Contezolid (Synonyms: MRX-I) |
| Catalog No.GC62706 |
Contezolid (MRX-I), a new and orally active oxazolidinone, is an antibiotic in study for complicated skin and soft tissue infections (cSSTI) caused by resistant Gram-positive bacteria.
Products are for research use only. Not for human use. We do not sell to patients.
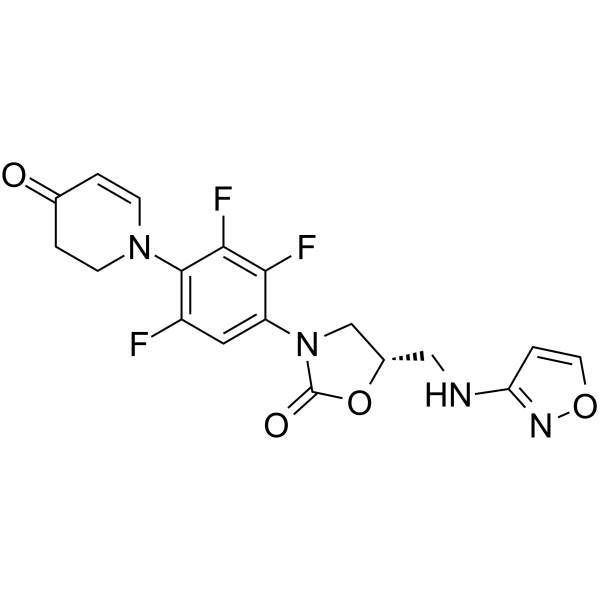
Cas No.: 1112968-42-9
Sample solution is provided at 25 µL, 10mM.
Contezolid (MRX-I), a new and oraly active oxazolidinone, is an antibiotic in study for complicated skin and soft tissue infections (cSSTI) caused by resistant Gram-positive bacteria. Contezolid (MRX-I) markedly reduces potential for myelosuppression and monoamine oxidase inhibition (MAOI)[1][2].
Contezolid (MRX-I) is highly potent against all Grampositive clinical isolates of staphylococci, streptococci, and enterococci, including MDR organisms such as MRSA, methicilline-resistant Streptococcus epidermidis (MRSE), penicillin-resistant Streptococci (PRSP), and VRE[2].
Oral absorption of Contezolid (MRX-I) occurrs rapidly in mouse, rat, and dog, with peak plasma concentrations observed at 0.5-2.6 h postdose. In mouse, rat, and dog, respectively, PK parameters are determined as follows: dose-normalized Cmax/dose was 524, 1065, and 259 ng/mL/(mg/kg); dose-normalized AUC0-t/dose was 1654, 3703, and 1664 ng•h/mL/(mg/kg); T1/2 is 1, 1.5, and 3 h; and the oral bioavailability is 69%, 109%, and 37%[2].Contezolid (MRX-I) exhibits no obvious toxicity[2].Contezolid (MRX-I, 100 mg/kg, once daily) significantly reduced the bacterial load in lungs compared to the untreated early and late controls[3].
[1]. Junzhen Wu, et al. Evaluation of the Effect of Contezolid (MRX-I) on the Corrected QT Interval in a Randomized, Double-Blind, Placebo- and Positive-Controlled Crossover Study in Healthy Chinese Volunteers. Antimicrob Agents Chemother. 2020 May 21;64(6):e02158-19.
[2]. Mikhail F Gordeev, et al. New potent antibacterial oxazolidinone (MRX-I) with an improved class safety profile. J Med Chem. 2014 Jun 12;57(11):4487-97.
[3]. Carolyn Shoen, et al. In Vitro and In Vivo Activities of Contezolid (MRX-I) against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2018 Jul 27;62(8):e00493-18.
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















