Marimastat (Synonyms: BB-2516, KB-R8898) |
| Katalog-Nr.GC14099 |
A broad-spectrum inhibitor of MMPs and TACE
Products are for research use only. Not for human use. We do not sell to patients.
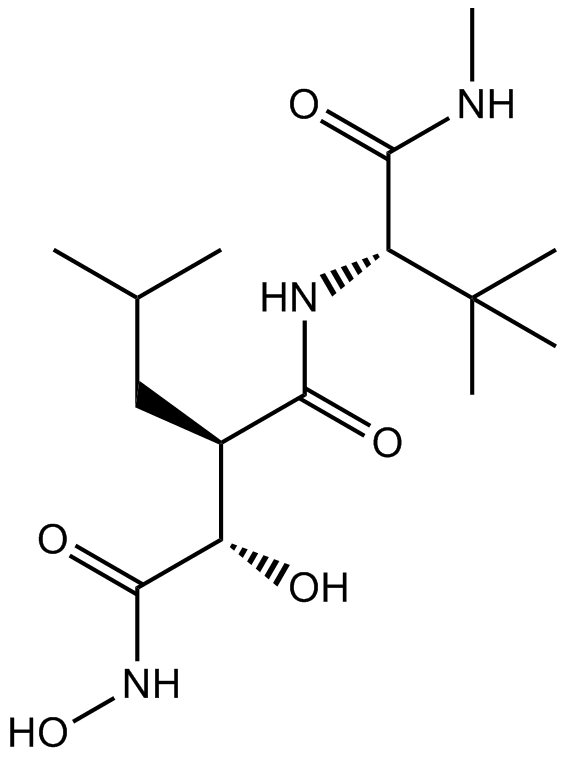
Cas No.: 154039-60-8
Sample solution is provided at 25 µL, 10mM.
Marimastat is a broad range inhibitor of Matrix metalloproteinases with IC50 values of 5, 6, 13, 3 and 9 nM for MMP-1, MMP-2, MMP-7 , MMP-9 and MMP-14 [1].
Marimastat is an orally bioavailable inhibitor with an absolute bioavailability of 20%–50% in preclinical studies, which can bind covalently to the zinc atom of the MMP-active site by a collagen-mimicking hydroxamate structure [1].
A reduction in the size and number of metastatic foci in treated compared with the control animals was demonstrated by experimental metastases models against lung and breast cancer. The studies were carried out at doses of 100–500 mg/kg per day, and the agent induced gastrointestinal toxicity and weight loss, as well as hemorrhage, fibrosis, inflammation, and necrosis at particular ankle and knee tissues. Single oral doses of up to 800 mg were well tolerated and did not lead to obvious toxicity. Peak plasma concentrations can be detected within 1.5–3 hours after oral administration, and the elimination half-life was estimated as a range of 8–10 hours. No plasma accumulation was detected after an oral doses of 50–200 mg in continuous administration twice a day for 6 consecutive days [2,3].
Pharmacological studies demonstrated that marimastat is well absorbed from the gastrointestinal tract and exhibits a linear pharmacokinetic behavior. The minimum plasma concentration was found after exceeding 10 mg doses twice a day, which were sixfold greater than the required for inhibition of MMP in vitro. Complain to the patients treated with gemcitabine, the most effective chemotherapeutic agent against the nonmetastatic pancreatic cancer, the patients who received high doses of marimastat had a 1-year survival rates. It is encouraging that the patients with unresectable gastric cancer who were treated with marimastat show a modest increase in survival [1,2].
References:
1.Hidalgo M, Eckhardt SG, Development of matrix metalloproteinase inhibitors in cancer therapy. JOURNAL OF THE NATIONAL CANCER INSTITUTE. 2001, 93(3):178-193.
2.Coussens LM, Fingleton B , Matrisian LM , Cancer therapy - Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. SCIENCE, 295 (5564): 2387-2392.
3.Van Wijngaarden J , Snoeks TJA , van Beek E, et al . An in vitro model that can distinguish between effects on angiogenesis and on established vasculature: Actions of TNP-470, marimastat and the tubulin-binding agent Ang-510. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, 2000, 391 (2): 1161-1165.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















