DG-051 |
| Catalog No.GC18471 |
DG-051 is a potent, orally bioavailable leukotriene A4 hydrolase (LTA4H) inhibitor (Kd = 25 nM; IC50 = 47 nM), the enzyme that catalyzes the rate-determining step in the synthesis of LTB4 .
Products are for research use only. Not for human use. We do not sell to patients.
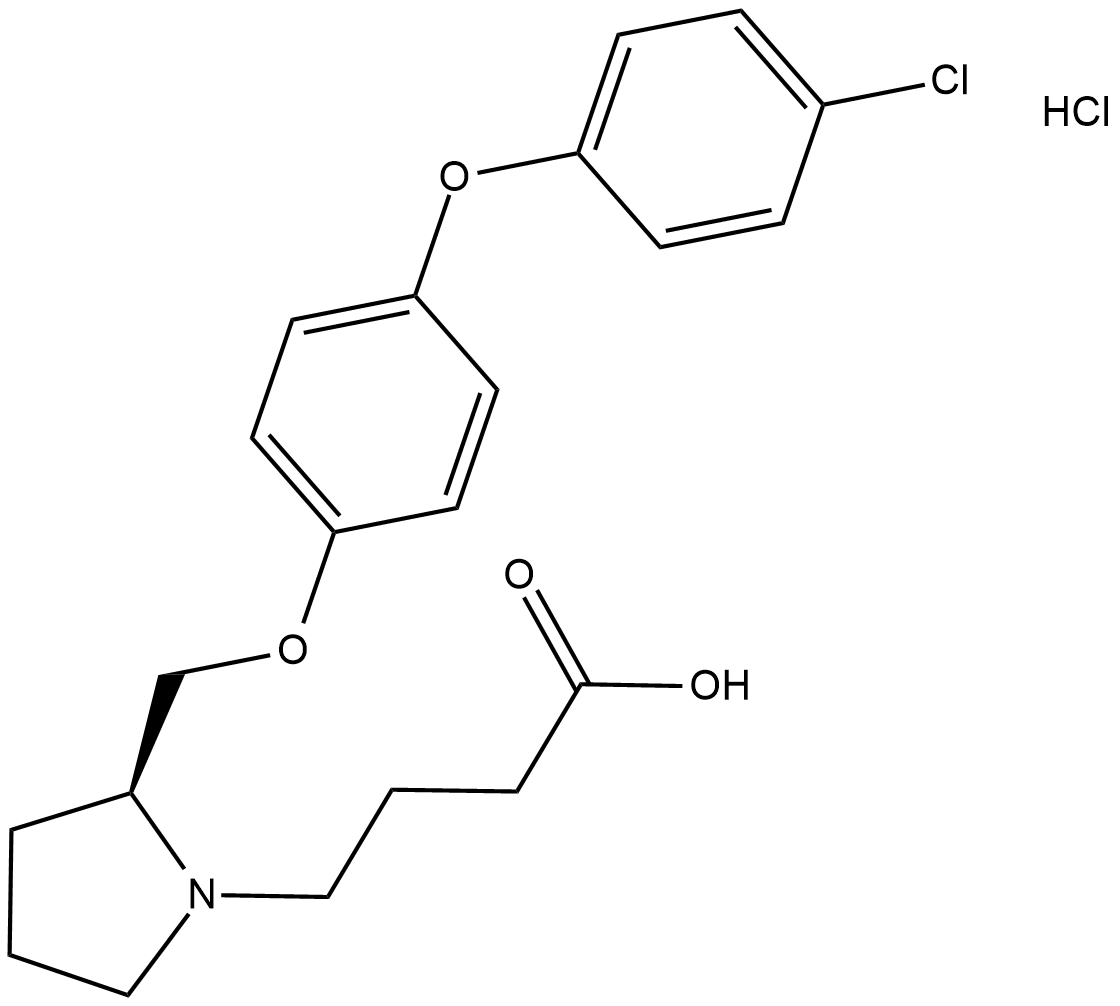
Cas No.: 929915-58-2
Sample solution is provided at 25 µL, 10mM.
DG-051 is a potent, orally bioavailable leukotriene A4 hydrolase (LTA4H) inhibitor (Kd = 25 nM; IC50 = 47 nM), the enzyme that catalyzes the rate-determining step in the synthesis of LTB4 . However, it also inhibits the aminopeptidase activity of LTA4H with IC50 values of 72 and 150 nM when tested using the substrates L-alanine p-nitroanaline and prolyl-glycyl-proline peptide , respectively, potentially leading to PGP accumulation in vivo. DG-051 does not show any inhibitory activity against a panel of more than 50 additional aminopeptidases, ion channels, or HERG.
References:
[1].Sandanayaka, V., Mamat, B., Mishra, R.K., et al. Discovery of 4-[(2S)-2-{[4-(4-chlorophenoxy)phenoxy]methyl}-1-pyrrolidinyl]butanoic acid (DG-051) as a novel leukotriene A4 hydrolase inhibitor of leukotriene B4 biosynthesis J. Med. Chem. 53(2), 573-585 (2010).
[2]. Low, C.M., Akthar, S., Patel, D.F., et al. The development of novel LTA4H modulators to selectively target LTB4 generation Sci. Rep. 7, 44449 (2017).
Average Rating: 5 (Based on Reviews and 33 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















