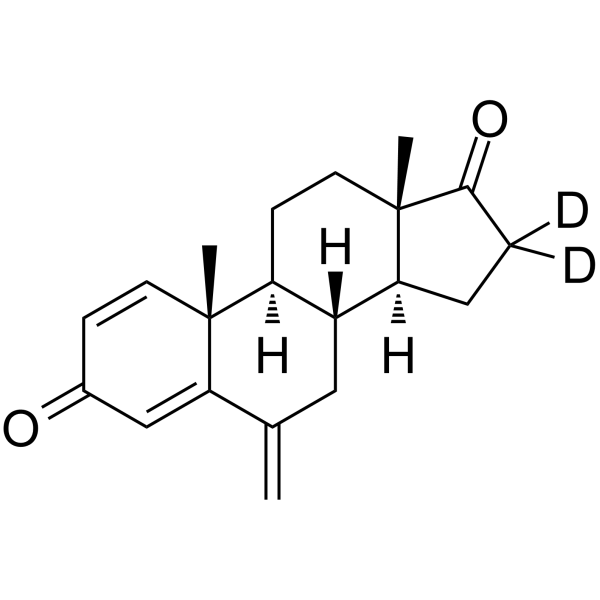
Exemestane-D2 (Synonyms: FCE 24304-D2; EXE-D2) |
| Catalog No.GC63755 |
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
Exemestane-D2 (FCE 24304-D2) is the deuterium labeled Exemestane. Exemestane (FCE 24304) is a selective, irreversible and orally active steroidal aromatase inhibitor with IC50s of 30 nM and 40 nM for human placental and rat ovarian aromatase, respectively. Exemestane can be used for hormone-dependent breast cancer research[1][2].
Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1].
[1]. Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216. [2]. Di Salle, E., et al., Novel aromatase and 5 alpha-reductase inhibitors. J Steroid Biochem Mol Biol, 1994. 49(4-6): p. 289-94.;Miki, Y, et al. Effects of aromatase inhibitors on human osteoblast and osteoblast-like cells: a possible androgenic bone protective effects induced by exemestane. Bone. 2004 Sep 1;10(17):5717-23.;Goss, P.E., et al., Effects of the steroidal aromatase inhibitor exemestane and the nonsteroidal aromatase inhibitor letrozole on bone and lipid metabolism in ovariectomized rats. Clin Cancer Res, 2004. 10(17): p. 5717-23.;Zaccheo, T., D. Giudici, and E. Di Salle, Inhibitory effect of combined treatment with the aromatase inhibitor exemestane and tamoxifen on DMBA-induced mammary tumors in rats. J Steroid Biochem Mol Biol, 1993. 44(4-6): p. 677-80.
Average Rating: 5 (Based on Reviews and 14 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















