Prostaglandin F2α Ethanolamide MaxSpec® Standard |
| Catalog No.GC41408 |
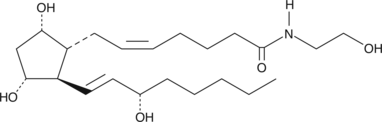
Prostaglandin F2α ethanolamide (PGF2α-EA) is produced by cyclooxygenase 2 (COX-2) metabolism of the endogenous cannabinoid, arachidonoyl ethanolamide, found in brain, liver, and other mammalian tissues.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 353787-70-9
Sample solution is provided at 25 µL, 10mM.
Prostaglandin F2α ethanolamide (PGF2α-EA) is produced by cyclooxygenase 2 (COX-2) metabolism of the endogenous cannabinoid, arachidonoyl ethanolamide , found in brain, liver, and other mammalian tissues. AEA can be metabolized directly by the sequential action of COX-2 and specific PG synthases to produce ethanolamide congeners of the classical PGs. PGF2α-EA has also been reported to be biosynthesized by this mechanism when AEA was infused into the lung and liver of living mice. PGF2α-EA is a potent dilator (EC50 = 58 nM) of the cat iris sphincter, which is a model system for testing potential intraocular hypotensive agents.PGF2α-EA MaxSpec® standard is a quantitative grade standard of PGF2α-EA that has been prepared specifically for mass spectrometry or any application where quantitative reproducibility is required. The solution has been prepared gravimetrically and is supplied in a deactivated glass ampule sealed under argon. The concentration was verified by comparison to an independently prepared calibration standard. This PGF2α-EA MaxSpec® standard is guaranteed to meet identity, purity, stability, and concentration specifications and is provided with a batch-specific certificate of analysis. Ongoing stability testing is performed to ensure the concentration remains accurate throughout the shelf life of the product. Note: The amount of solution added to the vial is in excess of the listed amount. Therefore, it is necessary to accurately measure volumes for preparation of calibration standards. Follow recommended storage and handling conditions to maintain product quality.
Average Rating: 5 (Based on Reviews and 15 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















