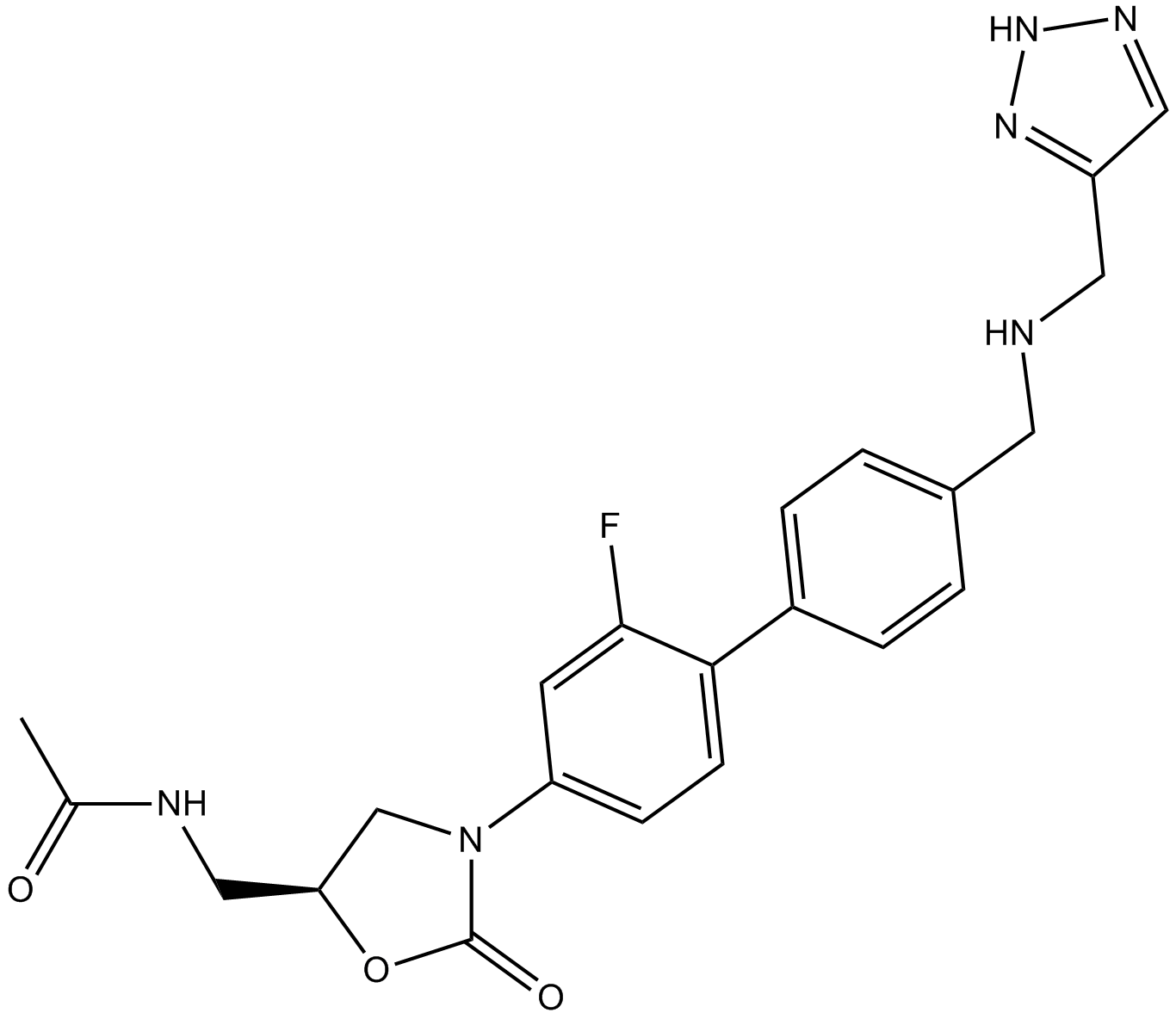
Radezolid (Synonyms: Rx-01_667, RX-1741) |
| Catalog No.GC13613 |
An oxazolidinone antibiotic
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 869884-78-6
Sample solution is provided at 25 µL, 10mM.
Radezolid Description:
MIC90: Radezolid was approximately four-times more potent than linezolid against MRSA, with MIC90 of 0.5 mg/l and 2.0 mg/l, respectively [1].
Radezolid is an investigational oxazolidinone with excellent in vitro and in vivo activity against a variety of Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA). Effcacy has been attributed to the finding that radezolid accumulates in vitro in macrophages, polymorphonuclear leukocytes (PMNs), epithelial and endothelial cells.
In vitro: A study found that radezolid accumulated to similar levels (~10-fold) in all cell types (human keratinocytes, endothelial cells, bronchial epithelial cells, osteoblasts, macrophages, and rat embryo fibroblasts). At equivalent weight concentrations, radezolid proved consistently 10-fold more potent than linezolid in all these models, irrespective of the bacterial species and resistance phenotype or of the cell type infected. These data suggest the potential interest of radezolid for recurrent or persistent infections where intracellular foci play a determinant role [2].
In vivo: When administered at 50 mg/kg, radezolid and linezolid showed comparable reductions in bacterial burden 24 hours after inoculation. Area under the curve (AUC) analysis of tissue concentrations demonstrated that radezolid accumulated 2.4-fold in infected thighs when compared to non-infected thigh tissue (table). Linezolid showed no accumulation in infected thighs [3].
Clinical trial: Radezolid (INN, codenamed RX-1741) is developed by Rib-X Pharmaceuticals, Inc. for the treatment of serious multi-drug–resistant infections. Radezolid has completed two phase-II clinical trials. One of these clinical trials was for uncomplicated skin and skin-structure infections (uSSSI) and the other clinical trial was for community acquired pneumonia (CAP) (http://en.wikipedia.org/wiki/Radezolid)..
Reference:
[1] Laura Lawrence, Paul Danese, Joe DeVito, Francois Franceschi, and Joyce Sutcliffe. In Vitro Activities of the Rx-01 Oxazolidinones against Hospital and Community Pathogens. Antimicrob Agents Chemother. 2008; 52(5): 1653–1662.
[2] Lemaire S, Kosowska-Shick K, Appelbaum PC, Verween G, Tulkens PM, Van Bambeke F. Cellular pharmacodynamics of the novel biaryloxazolidinone radezolid: studies with infected phagocytic and nonphagocytic cells, using Staphylococcus aureus, Staphylococcus epidermidis, Listeria monocytogenes, and Legionella pneumophila. Antimicrob Agents Chemother. 2010;54(6):2549-59.
[3] Burak E, Bortolon E, Molstad D, Jing H and Wu Y. Radezolid, a novel oxazolidinone, accumulates in infected thigh tissue. Post A1-1938. 49th ICAAC San Francisco, CA, USA September 12-15, 2009
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















