10(S)-PAHSA |
| Catalog No.GC40318 |
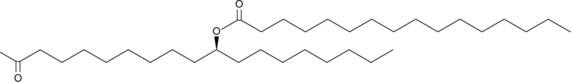
10(S)-PAHSA is a stereoisomer of 10-PAHSA, an endogenous lipid that belongs to a collection of branched fatty acid esters of hydroxy fatty acids (FAHFAs).
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
10(S)-PAHSA is a stereoisomer of 10-PAHSA , an endogenous lipid that belongs to a collection of branched fatty acid esters of hydroxy fatty acids (FAHFAs). It is a FAHFA in which palmitic acid is esterified to 10-hydroxy stearic acid. Among the FAHFA family members, PAHSAs are the most abundant in the adipose tissue of glucose tolerant AG4OX mice, which overexpress the Glut4 glucose transporter specifically in adipose tissue. A study using synthesized (R)- and (S)- stereoisomers of 9-PAHSA reported that the 9(R)-PAHSA is the predominant form that accumulates in adipose tissues in vivo. Also, cell lines favor the production of 9(R)-PAHSA and carboxyl ester lipase selectively hydrolyzes 9(S)-PAHSA .The use of this optically-active FAHFA product (the "Product") is covered by U.S. Patent No. 10,240,025 and corresponding foreign counterpart applications.
Average Rating: 5 (Based on Reviews and 31 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















