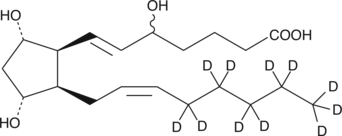
(±)5-iPF2α-VI-d11 |
| Catalog No.GC46263 |
An internal standard for the quantification of (±)5iPF2αVI
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 936565-17-2
Sample solution is provided at 25 µL, 10mM.
(±)5-
1.Morrow, J.D., Hill, K.E., Burk, R.F., et al.A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanismProc. Natl. Acad. Sci. U.S.A.87(23)9383-9387(1990) 2.Parchmann, S., and Mueller, M.J.Evidence for the formation of dinor isoprostanes E1 from α-linolenic acid in plantsThe Journal of Biological Chemisty27332650-32655(1998) 3.Delanty, N., Reilly, M., Pratico, D., et al.8-Epi PGF2α: Specific analysis of an isoeicosanoid as an index of oxidant stress in vivoBritish Journal of Clinical Pharmacology4215-19(1996) 4.Reilly, M.P., Barry, P., Lawson, J.A., et al.Urinary 8-epi PGF2α: An index of oxidant stress in vivoFibrinolysis & Proteolysis1181-84(1997) 5.Reilly, M.P., Pratico, D., Delanty, N., et al.Increased formation of distinct F2 isoprostanes in hypercholesterolemiaCirculation982822-2828(1998) 6.Li, H., Lawson, J.A., Reilly, M., et al.Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F2-isoprostanes in human urineProceedings of the National Academy of Sciences of the United States of America96(23)13381-13386(1999) 7.Lawson, J.A., Li, H., Rokach, J., et al.Identification of two major F2 isoprostanes, 8,12-iso- and 5-epi-8,12-iso-isoprostane F2α-VI, in human urineThe Journal of Biological Chemisty27329295-29301(1998) 8.PraticÒ, D., Barry, O.P., Lawson, J.A., et al.IPF2α-I: An index of lipid peroxidation in humansProceedings of the National Academy of Sciences of the United States of America953449-3454(1998)
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















