Anthranilic Acid (Synonyms: NSC 144, NSC 40929) |
| Catalog No.GC41593 |
Anthranilic Acid is a potential precursor in the synthesis of quinazolinones, including methaqualone.[1]
Products are for research use only. Not for human use. We do not sell to patients.
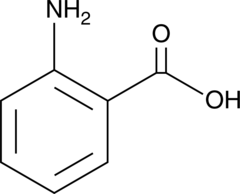
Cas No.: 118-92-3
Sample solution is provided at 25 µL, 10mM.
Anthranilic Acid is a potential precursor in the synthesis of quinazolinones, including methaqualone.[1] For this reason, anthranilic acid, its esters, and its salts are categorized as List I chemicals with the Drug Enforcement Administration in the United States. Anthranilic acid is also a natural antimicrobial metabolite produced by a number of bacterial strains.[2] This product is intended for research and forensic applications.
Reference:
[1]. Soliman, F.S., Shafik, R.M., and Elnenaey, E.A. Synthesis of methaqualone and its diphasic titration in pure and tablet forms. Journal of Pharmaceutical Sciences 67(3), 411-413 (1978).
[2]. Hund, H.K., de Beyer, A., and Lingens, F. Microbial metabolism of quinoline and related compounds. VI. Degradation of quinaldine by Arthrobacter sp. Biological Chemistry of Hoppe-Seyler 371(10), 1005-1008 (1990).
Average Rating: 5 (Based on Reviews and 9 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















