Chlorpheniramine Maleate (Synonyms: (±)-Chlorpheniramine, Chlorprophenpyridamine) |
| Catalog No.GC16047 |
Histamine H1 receptor antagonist
Products are for research use only. Not for human use. We do not sell to patients.
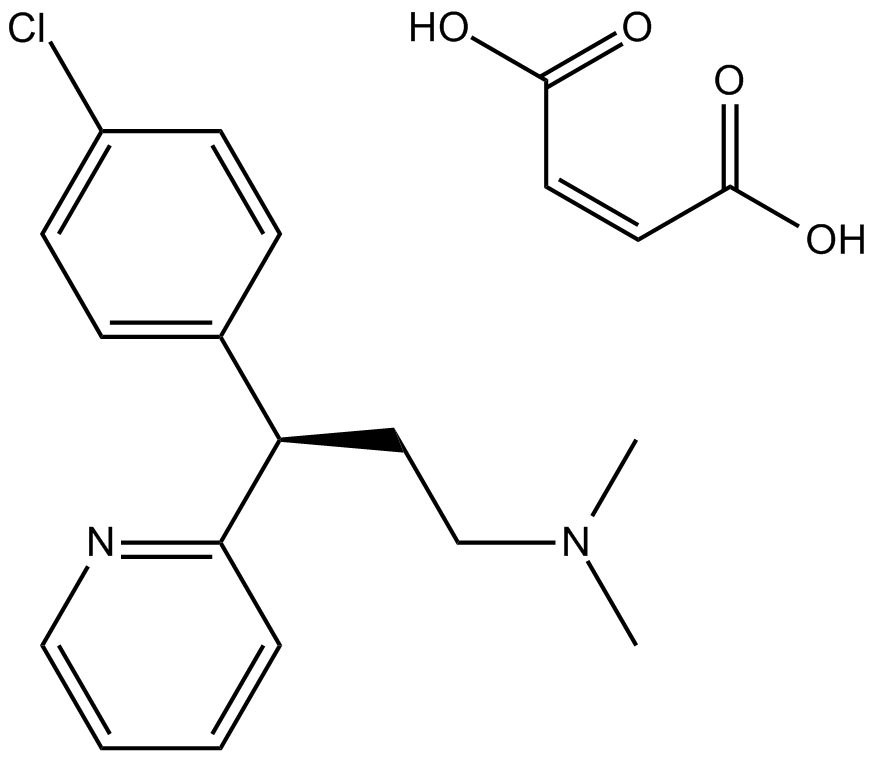
Cas No.: 113-92-8
Sample solution is provided at 25 µL, 10mM.
Chlorpheniramine maleate is an histamine H1 receptor antagonist with IC50 of 12 nM.Target: Histamine H1 ReceptorChlorpheniramine inhibits the proliferation of MCF-7, MDA-MB 231, and Ehrlich cells in a dose-response manner, and significantly reduces the ornithine decarboxylase mRNA translation by 50%-70% at the 250 μM [1]. Chlorpheniramine displaces of [3H]pyrilamine from human histamine receptor subtype 1 expressed in CHO cells with IC50 of 66 nM. Chlorpheniramine displays antimalarial activity against CQS strain (D6) and MDR strain (Dd2) of P. falciparum with IC50 of 61.2 uM and 3.9 uM, respectively. Chlorpheniramine displays cytotoxicity against the proliferation of concanavalin A-induced murine splenic lymphocytes with IC50 of 33.4 μM [2].Oral administration of Chlorpheniramine inhibits histamine-induced mortality in guinea pigs with an ED50 of 0.17 mg/kg [3]. Oral administration of Chlorpheniramine (10 mg/kg) significantly inhibits short-duration scratching in BALB/c mice stimulated by ovalbumin active cutaneous anaphylaxis and in ICR mice subcutaneously injected with histamine, but not long-duration scratching seen in NC/Nga mice, in contrast to that of dexamethasone or tacrolimus [4].
References:
[1]. Medina, M.A., et al., Chlorpheniramine inhibits the synthesis of ornithine decarboxylase and the proliferation of human breast cancer cell lines. Breast Cancer Res Treat, 1995. 35(2): p. 187-94.
[2]. Kelly, J.X., et al., Design, synthesis, and evaluation of 10-N-substituted acridones as novel chemosensitizers in Plasmodium falciparum. Antimicrob Agents Chemother, 2007. 51(11): p. 4133-40.
[3]. Iemura, R., et al., Synthesis of 2-(4-substituted-1-piperazinyl)benzimidazoles as H1-antihistaminic agents. J Med Chem, 1986. 29(7): p. 1178-83.
[4]. Takano, N., I. Arai, and M. Kurachi, Analysis of the spontaneous scratching behavior by NC/Nga mice: a possible approach to evaluate antipruritics for subjects with atopic dermatitis. Eur J Pharmacol, 2003. 471(3): p. 223-8.
Average Rating: 5 (Based on Reviews and 33 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















