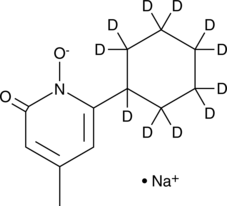
Ciclopirox-d11 (sodium salt) (Synonyms: HOE 296b-d11) |
| Catalog No.GC45764 |
A neuropeptide with diverse biological activities
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: N/A
Sample solution is provided at 25 µL, 10mM.
Ciclopirox-d11 (sodium salt) is intended for use as an internal standard for the quantification of ciclopirox by GC- or LC-MS. Ciclopirox is an iron chelator, antifungal, and anticancer agent.1,2,3,4 It inhibits the iron-dependent enzyme prolyl hydroxylase 2 (PHD2; IC50 = 1.58 μM), an effect that is reduced in the presence of iron.1 It stabilizes hypoxia-inducible factor-α (HIF-1α) under normoxic conditions in rat glomus cells when used at a concentration of 5 μM.5 Ciclopirox is active against clinical isolates of T. rubrum, T. mentagrophytes, and C. albicans (MICs = 0.03-0.5, 0.03-0.5, and 0.06-0.5 μg/ml, respectively) and inhibits growth of T. mentagrophytes on porcine skin ex vivo when applied topically.2,3 It inhibits proliferation of Rh30, HT-29, and MDA-MB-231 cells in a concentration-dependent manner and halts the cell cycle at the G1/G0 phase and induces apoptosis in Rh30 cells.4 Ciclopirox (25 mg/kg) reduces tumor growth in an MDA-MB-231 mouse xenograft model. Formulations containing ciclopirox have been used in the topical treatment of fungal infections.
|1. Aowicki, D., and Huczynski, A. Structure and antimicrobial properties of monensin A and its derivatives: Summary of the achievements. Biomed. Res. Int. 2013:742149, (2013).|2. Jo Siu, W.J., Tatsumi, Y., Senda, H., et al. Comparison of in vitro antifungal activities of efinaconazole and currently available antifungal agents against a variety of pathogenic fungi associated with onychomycosis. Antimicrob. Agents Chemother. 57(4), 1610-1616 (2013).|3. Ceschin-Roques, C.G., H•nel, H., Pruja-Bougaret, S.M., et al. Ciclopirox nail lacquer 8%: In vivo penetration into and through nails and in vitro effect on pig skin. Skin Pharmacol. 4(2), 89-94 (1991).|4. Zhou, H., Shen, T., Luo, Y., et al. The antitumor activity of the fungicide ciclopirox. Int. J. Cancer 127(10), 2467-2477 (2010).|5. Baby, S.M., Roy, A., Mokashi, A.M., et al. Effects of hypoxia and intracellular iron chelation on hypoxia-inducible factor-1α and -1β in the rat carotid body and glomus cells. Histochem. Cell. Biol. 120(5), 343-352 (2003).
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















