Curcumin (Synonyms: Indian Saffron, Turmeric yellow) |
| Katalog-Nr.GC14787 |
Ein gelbes Pigment mit vielfältigen biologischen Aktivitäten.
Products are for research use only. Not for human use. We do not sell to patients.
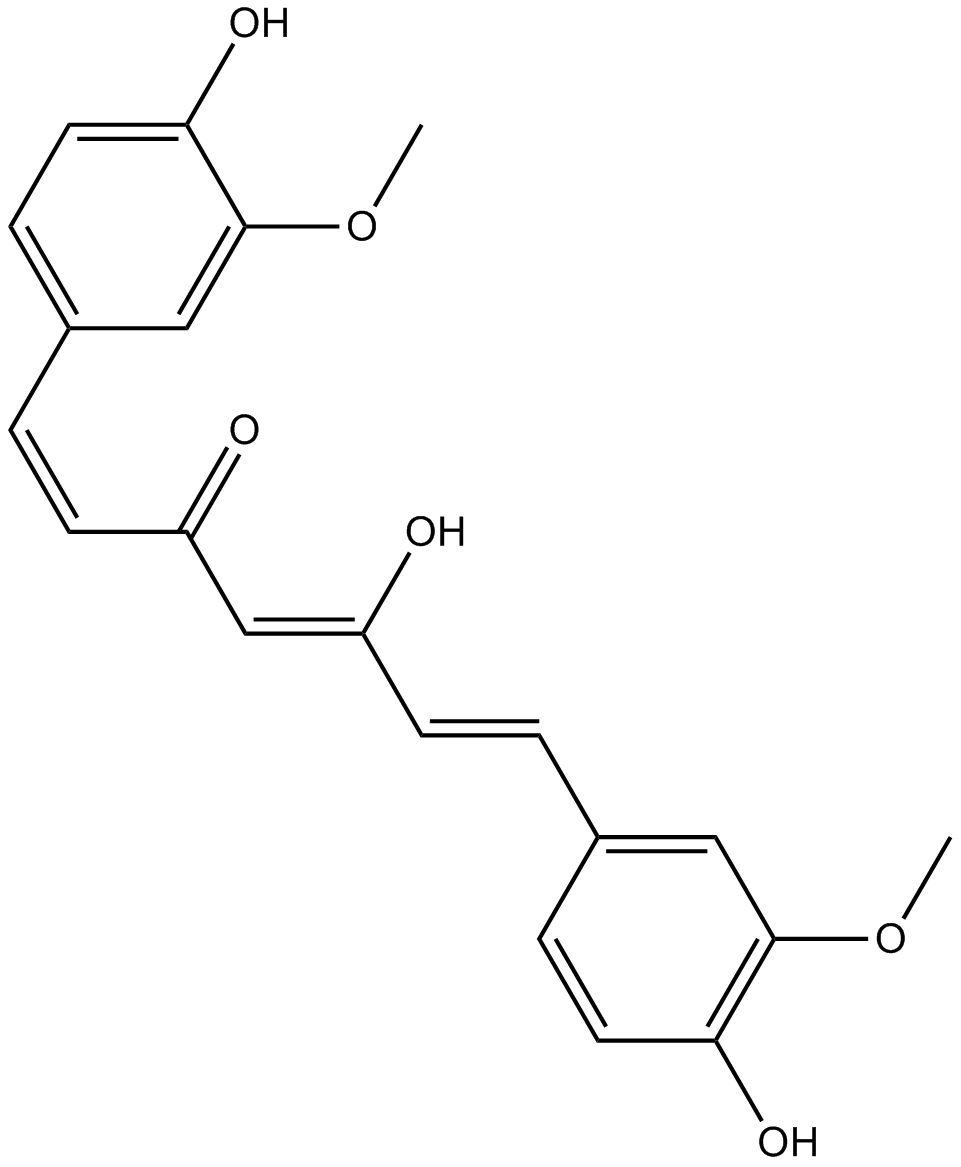
Cas No.: 458-37-7
Sample solution is provided at 25 µL, 10mM.
Curcumin is an inhibitor of tyrosinase with IC50 value of 47uM [1].
Curcumin is a natural compound with potency of anticancer. It is currently under clinical investigation for cancer chemoprevention. There is a variety of biochemical mechanisms of this anticancer function. The targets of Curcumin are involved in signaling pathways include transcription factors, growth factors,
inflammatory cytokines, receptors, and enzymes. Phase I trials have tested the toxicity and tolerability of Curcumin and found that Curcumin has no toxicities at doses up to 12 g/day. However, the bioavailability of Curcumin is quite poor. It is about ~1% after oral administration in phase I/II clinical trials and this hinders Curcumin‘s use in the clinic [2].
It is also reported that Curcumin reduces the generation of amloid beta (Aβ 40 and Aβ42) in SH-SY5Y neuroblastoma cells. It also down-regulates the expression of PS1 and GSK-3β in cells. All these cause the inhibition of Aβ formation and make Curcumin to be a potent therapeutic agent in AD [3].
References:
[1] Sachiko Shirota, Kouji Miyazaki, Ritsuo Aiyama, Minoru Ichioka and Teruo Yokokura. Tyrosinase inhibitors from crude drugs. Biol. Pharm. Bull. 1994, 17 (2): 266-269.
[2] Wungki Park, A.R.M Ruhul Amin, Zhuo Georgia Chen, and Dong M. Shin. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila). 2013, 6(5): 387–400.
[3] Zhang Xiong, Zhang Hongmei, SiLu, LiYu. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s disease. Pharmacological Reports. 2013, 63: 1101-1108.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















