Epothilone D (Synonyms: Desoxyepothilone B, NSC 73147) |
| Catalog No.GC17440 |
A microtubule-stabilizing agent
Products are for research use only. Not for human use. We do not sell to patients.
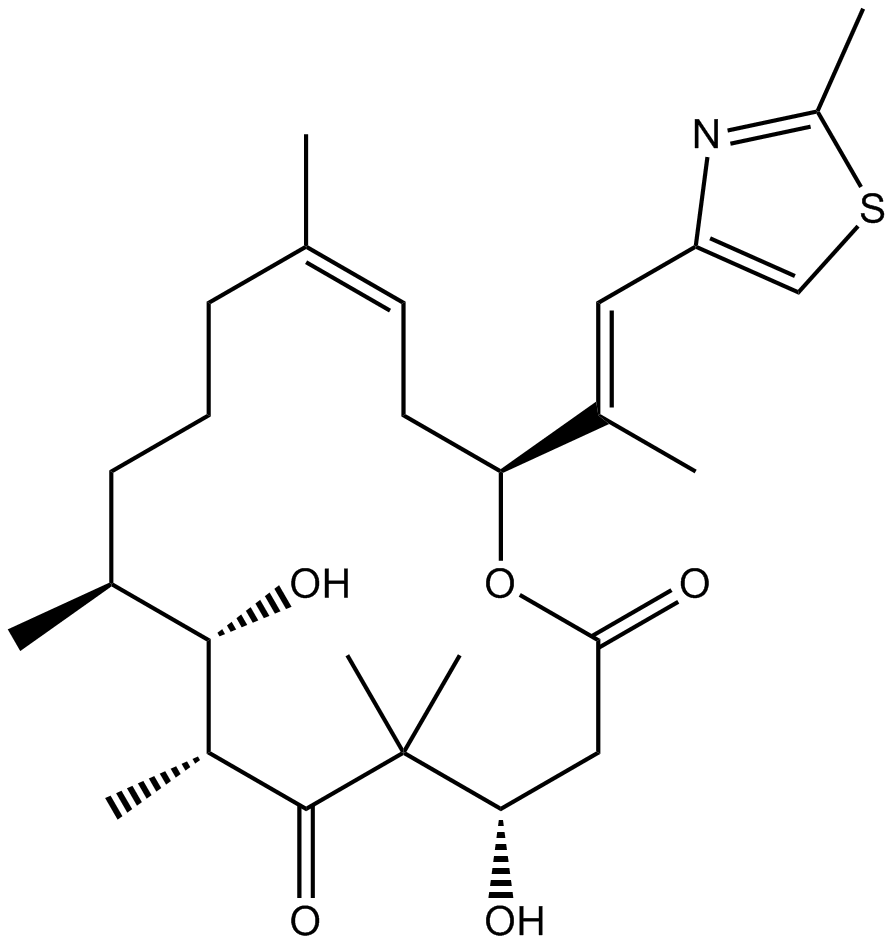
Cas No.: 189453-10-9
Sample solution is provided at 25 µL, 10mM.
IC50: 2.9 nM for MCF-7 cell line; 2.7 nM for KB-31 cell line; 9.5 nM for CCRF-CEM cell line
Drugs targeting tubulin are active in human malignant disease and are an essential component of medical treatment of these diseases. As a result, pharmaceutical research on compounds that interfere with tubulin function has concentrated on agents which might have enhanced efficacy or reduced toxicity. Epothilone D is a more potent microtubule stabilizer.
In vitro: Epothilone D is a more potent microtubule stabilizer in vitro than epothilone A or B. In vitro, Epothilone D showed potent cytotoxicity in a panel of human tumor cell lines, with similar potency to paclitaxel. It also showed definite advantage over paclitaxel in drug-resistant cell lines, and retained its cytotoxicity against a multidrug resistant cell line over-expressing P-glycoprotein [1].
In vivo: In vivo, antitumor efficacy of Epothilone D has been observed in both paclitaxel sensitive and resistant xenografts, as well as certain multidrug resistant xenografts including a doxorubinresistant CCRF-CEM leukemic cell xenograft [1].
Clinical trial: Epothilone D was well tolerated with manageable toxicity, favorable PK profile, and clinical activity. The maximum tolerated dose was determined to be 100 mg/m2 weekly 3-on/1-off. MTBF can be demonstrated in PBMCs of patients exposed to Epothilone D [1].
Reference:
[1] Konner J, Grisham RN, Park J, O'Connor OA, Cropp G, Johnson R, Hannah AL, Hensley ML, Sabbatini P, Mironov S, Danishefsky S, Hyman D, Spriggs DR, Dupont J, Aghajanian C. Phase I clinical, pharmacokinetic, and pharmacodynamic study of KOS-862 (Epothilone D) in patients with advanced solid tumors and lymphoma. Invest New Drugs. 2012 Dec;30(6):2294-302. doi: 10.1007/s10637-011-9765-7.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















