Firategrast |
| Catalog No.GC14804 |
Products are for research use only. Not for human use. We do not sell to patients.
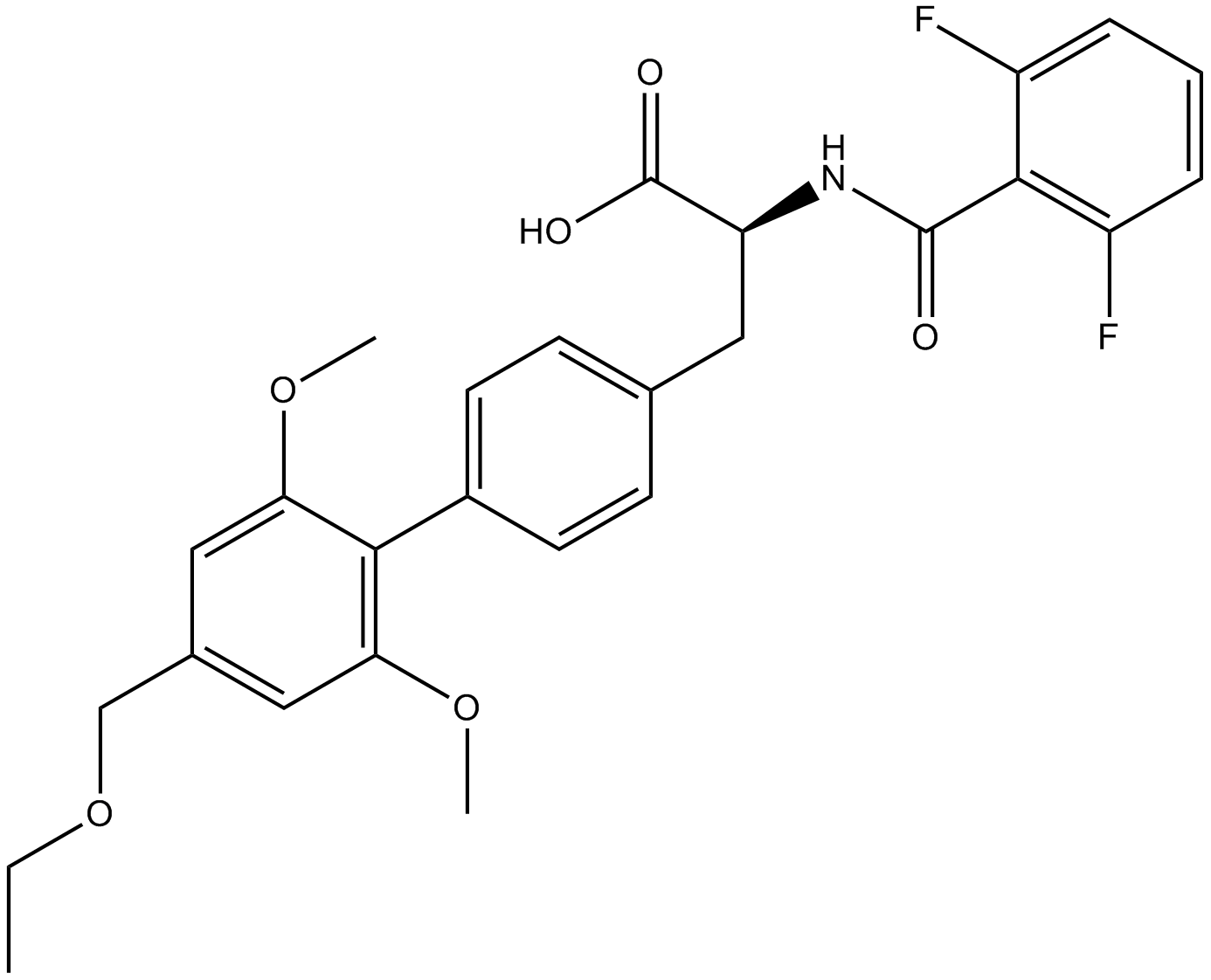
Cas No.: 402567-16-2
Sample solution is provided at 25 µL, 10mM.
Firategrast is a bioavailable small-molecule antagonist of α4β1 and α4β7 integrins [1].
The integrins are a sort of transmembrane receptors that modulate the signal transduction from ECM to the cell. They are associated with a lot of diseases such as cancer, inflammation and thrombotic diseases. Since part of the integrin is exposed to the cell outside and is easy to combine with the drug, integrins are thought to be attracted targets. There are many drugs target the integrins have been designed and generated, such as abciximab, tirofiban, lamifiban and natalizumab. Among these, firategrast is a drug for the treatment of multiple sclerosis (MS) which is found to be caused by the migration of leucocytes (such as monocytes, T cells, B cells and dendritic cells) into CNS. And the integrin α4β1 is found to take participate in the migration through activating the leucocytes [1, 2].
Firategrast has a much shorter half-life than natalizumab with about 2.5 hours to 4.5 hours. It is found to inhibit the binding of the integrins to the associated ligands, including vascular cell adhesion protein 1 and mucosal addressin cell adhesion molecule 1. In CNS, firategrast treatment caused moderate decreases of total lymphocyte count, lymphocyte subset count and the ratio of CD4 to CD8. In peripheral blood, firategrast treatment resulted in the increases of total lymphocyte count, all lymphocyte subset count as well as the peripheral CD34+ early haematopoietic progenitor cell count [1, 3].
Firategrast was well tolerated at the maximum doses of 1200 mg for men and 900 mg for women. Firategrast showed no side effects, such as PML or JC-virus reactivation, at these doses. In Phase I clinical trials, the administration of firategrast significantly reduced the cumulative number of new gadolinium-enhancing lesions in patients with relapsing remitting MS [1, 3].
References:
[1] Grove R A, Shackelford S, Sopper S, et al. Leukocyte counts in cerebrospinal fluid and blood following firategrast treatment in subjects with relapsing forms of multiple sclerosis. European Journal of Neurology, 2013, 20(7): 1032-1042.
[2] Prat A, Stüve O. Firategrast—natalizumab in a pill?. The Lancet Neurology, 2012, 11(2): 120-121.
[3] Miller D H, Weber T, Grove R, et al. Firategrast for relapsing remitting multiple sclerosis: a phase 2, randomised, double-blind, placebo-controlled trial. The Lancet Neurology, 2012, 11(2): 131-139.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















