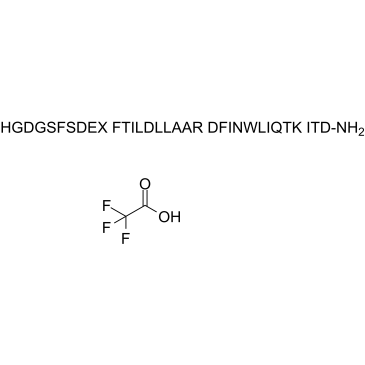
Apraglutide TFA (Synonyms: FE 203799 TFA) |
| Catalog No.GC35376 |
Apraglutide TFA (FE 203799 TFA), a synthetic 33-amino-acid peptide and a long-acting GLP-2 analogue, enhances adaptation and linear intestinal growth in a neonatal piglet model of short bowel syndrome with total resection of the ileum.
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
Apraglutide TFA (FE 203799 TFA), a synthetic 33-amino-acid peptide and a long-acting GLP-2 analogue, enhances adaptation and linear intestinal growth in a neonatal piglet model of short bowel syndrome with total resection of the ileum[1].
Apraglutide (FE 203799; 5 mg/kg/dose, subcutaneously, twice on days 0 and 4 postsurgery) treated piglets are healthy, have significant lower fecal fat and energy losses and exhibite intestinal lengthening, greater small-intestinal weight, longer villus height, and greater crypt depth on day 7[1]. Animal Model: NewbornDuroc piglets, 2-5 days old and weighing between 2-2.6 kg[1].
[1]. Slim GM, et al. Novel Long-Acting GLP-2 Analogue, FE 203799 (Apraglutide), Enhances Adaptation and Linear Intestinal Growth in a Neonatal Piglet Model of Short Bowel Syndrome with Total Resection of the Ileum. JPEN J Parenter Enteral Nutr. 2019 Jan 6.
Average Rating: 5 (Based on Reviews and 19 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















