JAK2/FLT3-IN-1 TFA |
| Catalog No.GC62665 |
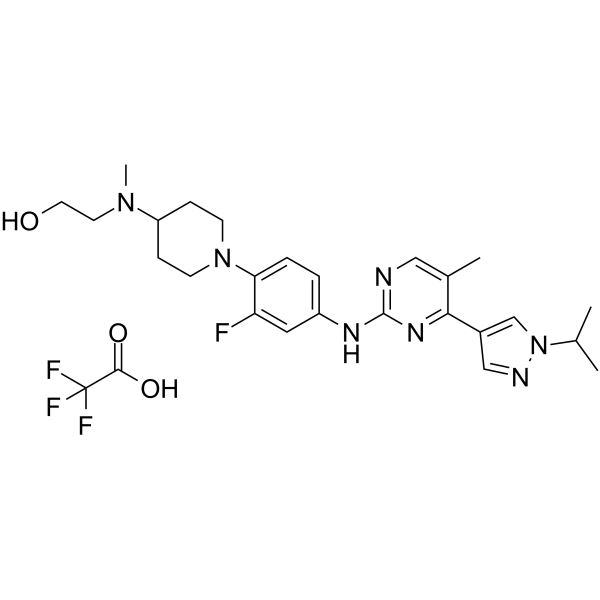
JAK2/FLT3-IN-1 (TFA) is a potent and orally active dual JAK2/FLT3 inhibitor with IC50 values of 0.7 nM, 4 nM, 26 nM and 39 nM for JAK2, FLT3, JAK1 and JAK3, respectively. JAK2/FLT3-IN-1 (TFA) has anti-cancer activity.
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
JAK2/FLT3-IN-1 (TFA) is a potent and orally active dual JAK2/FLT3 inhibitor with IC50 values of 0.7 nM, 4 nM, 26 nM and 39 nM for JAK2, FLT3, JAK1 and JAK3, respectively. JAK2/FLT3-IN-1 (TFA) has anti-cancer activity[1].
JAK2/FLT3-IN-1 (0.008-1 μM; for 2 hours) (TFA) down-regulates p-FLT3 in a dose-dependent manner[1].JAK2/FLT3-IN-1 (5-100 nM; for 2 hours) (TFA) has a dose-dependent effect on the induction of apoptosis in the MV4-11 cells[1].JAK2/FLT3-IN-1 (5-100 nM; for 2 hours) (TFA) strongly induces cell cycle arrest with a G1/G0 percentage of 85% at 100 nM in the MV4-11 cells[1].
JAK2/FLT3-IN-1 (30 and 60 mg/kg/day; p.o.; for 14 days) (TFA) exhibits significant antitumor effects[1].
[1]. Yang T, et al. Discovery of Potent and Orally Effective Dual JAK2/FLT3 Inhibitors for the Treatment of AcuteMyelogenous Leukemia and Myeloproliferative Neoplasms. J Med Chem. 2019 Oct 31.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















