1. Cell preparation
Adherent cells: It is recommended to plate the cells one day before transfection and use antibiotic-free medium to inoculate the cells so that they can reach 70-90% confluence the next day.
Suspension cells: It is recommended that the cell density reach 2-4×106/ml on the day of transfection.
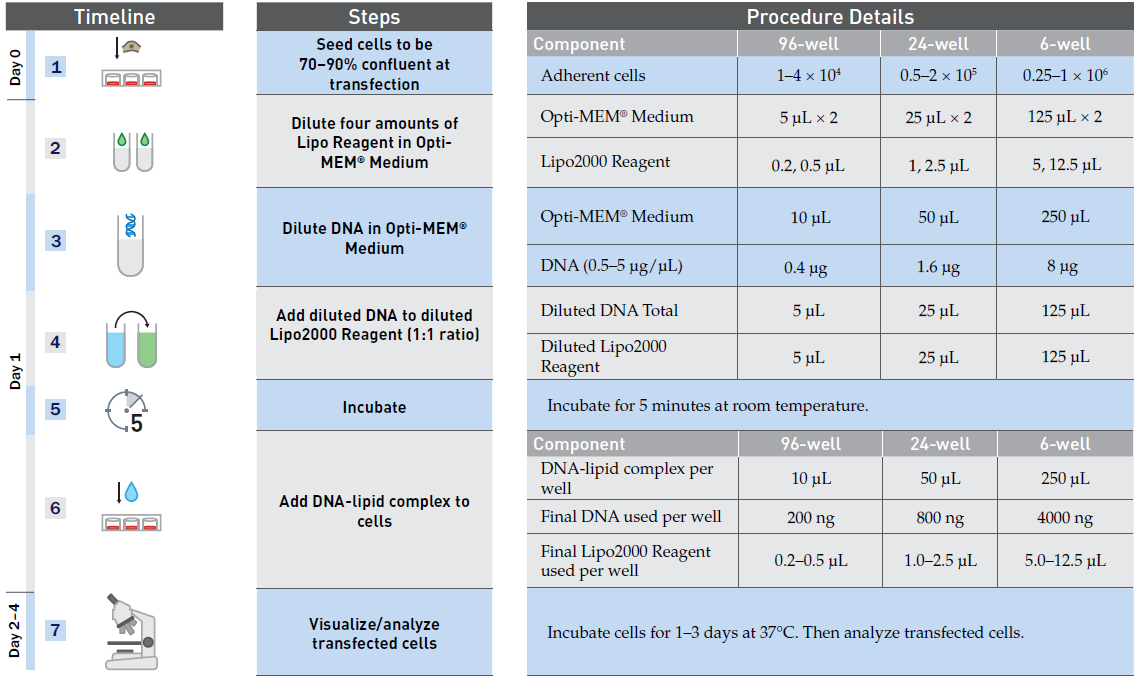
2.Lipo2000 DNA transfection reagent protocol
3. Co-transfection of plasmid DNA and siRNA
Lipo2000 reagent is suitable for simultaneous transfection of plasmid DNA and siRNA, adding 30pmol (~0.6μg) siRNA per 1μg DNA.
4.mRNA transfection
Lipo2000 reagent is suitable for transfecting mRNA. Taking a 24-well plate as an example, add 0.5–1μg of mRNA to each well.
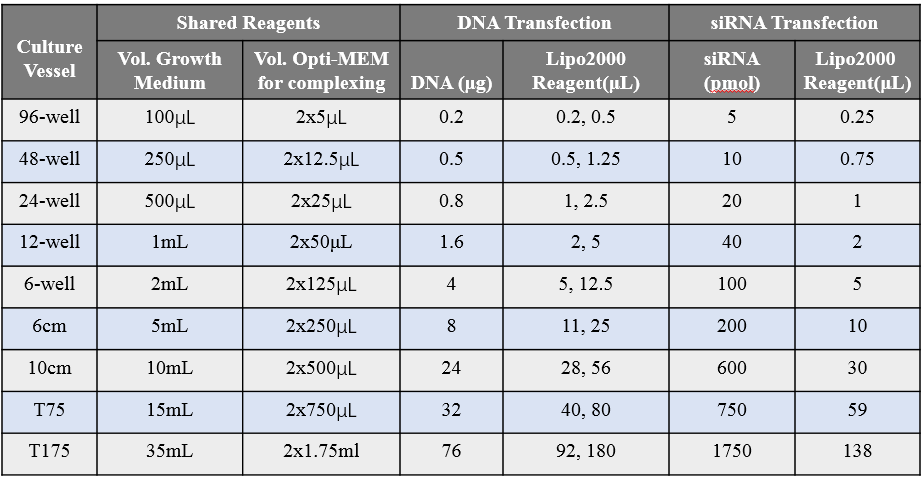
5. Recommended table of media, nucleic acids and Lipo2000 dosage for transfection in different cell culture plates
Precautions:
1. The cell status, DNA or siRNA in each experiment are different to a certain extent. It is recommended to explore the best conditions through preliminary experiments, especially the dosage of transfection reagent, and set up positive controls and negative controls at the same time.
2. Pay attention to the dosage ratio of liposomes and plasmids during transfection. Excessive liposomes will cause greater toxicity to the cells, leading to experimental failure. In order to achieve the highest transfection efficiency and lowest cytotoxicity, the ratio of DNA to Lipo2000 and cell density can be optimized. Generally, the ratio of DNA (μg) and Lipo2000 (μl) is optimized within the range of 1:0.5~1:5.
3. Cell status has a greater impact on the transfection effect. Cells should be in a good growth state during transfection. It is recommended that the cell density reach 70-80% during transfection.
4. For adherent cells, it is recommended to perform transfection 12-24 hours after plating. At this time, the cells are in good adhesion.
5. In order to improve the transfection efficiency, it is recommended to use antibiotic-free medium to inoculate cells when plating before transfection, and to replace the medium with low serum (2%), antibiotic-free medium 2 hours before transfection. Starvation treatment and replacement with complete culture medium 4-6 hours after transfection can greatly improve the transfection efficiency.
6. The DNA or RNA required for transfection must be of high purity and high quality to achieve higher transfection efficiency. The appropriate plasmid concentration for transfection is 0.5-5μg/μL, and there is no contamination by endotoxin, protein, RNA or other chemical substances.
7. The serum in the culture medium will affect the formation of liposome complexes. It is recommended to use serum-free Opti-MEM serum-reduced medium to configure the transfection system. The serum in the cell culture medium will not affect the transfection efficiency.
8. If you need to screen for stably transfected cell lines, it is recommended to inoculate the cells into fresh medium at a ratio of 1:10 or higher 24 hours after transfection, and change to selective medium for screening the next day. It is recommended to select corresponding drugs according to the resistance markers contained in different gene vectors. Commonly used markers in eukaryotic expression gene vectors include hygromycin and neomycin.





