N-(β-ketocaproyl)-L-Homoserine lactone (Synonyms: 3OC6-HSL) |
| Catalog No.GC17880 |
N-(β-ketocaproyl)-L-Homoserine lactone is a component of quorum regulatory sensing.
Products are for research use only. Not for human use. We do not sell to patients.
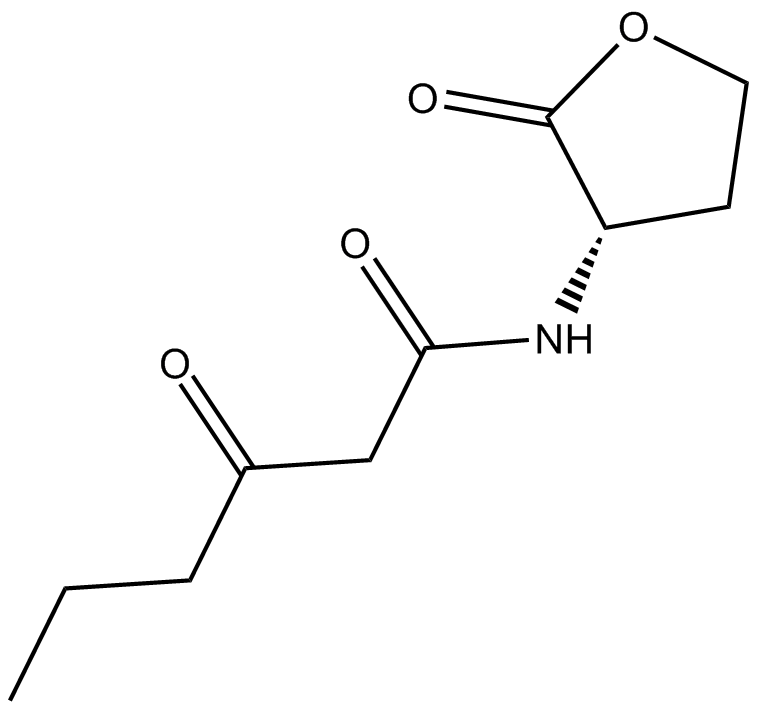
Cas No.: 143537-62-6
Sample solution is provided at 25 µL, 10mM.
Quorum sensing is a regulatory system used by bacteria for controlling gene expression in response to increasing cell density.[1] This regulatory process manifests itself with a variety of phenotypes including biofilm formation and virulence factor production.[2] Coordinated gene expression is achieved by the production, release, and detection of small diffusible signal molecules called autoinducers. The N-acylated homoserine lactones (AHLs) comprise one such class of autoinducers, each of which generally consists of a fatty acid coupled with homoserine lactone (HSL). Regulation of bacterial quorum sensing signaling systems to inhibit pathogenesis represents a new approach to antimicrobial therapy in the treatment of infectious diseases.[3] AHLs vary in acyl group length (C4-C18), in the substitution of C3 (hydrogen, hydroxyl, or oxo group), and in the presence or absence of one or more carbon-carbon double bonds in the fatty acid chain. These differences confer signal specificity through the affinity of transcriptional regulators of the LuxR family.[4] In one of the most-studied quorum-sensing systems in gram-negative bacteria, the LuxI AHL synthase catalyzes the production of N-(β-ketocaproyl)-L-homoserine lactone utilizing S-adenosylmethionine and hexanoyl-acyl carrier protein as reaction substrates in the marine bioluminescence bacterium V. fischeri.[5] At increased populations of the bacteria, localized higher concentrations of 3-O-C6-HSL, an endogenous ligand to transcriptional factor LuxR, leads to increased production of both the AHL synthase and proteins responsible for bioluminescence.[1] Numerous other species of bacteria also employ N-(β-ketocaproyl)-L-homoserine lactone in cell-to-cell communication.[6],[7],[8],[9]
Reference:
[1]. González, J.E., and Keshavan, N.D. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 70(4), 859-875 (2006).
[2]. Gould, T.A., Herman, J., Krank, J., et al. Specificity of acyl-homoserine lactone syntheses examined by mass spectrometry. J. Bacteriol. 188(2), 773-783 (2006).
[3]. Cegelski, L., Marshall, G.R., Eldridge, G.R., et al. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 6(1), 17-27 (2008).
[4]. Penalver, C.G.N., Morin, D., Cantet, F., et al. Methylobacterium extorquens AM1 produces a novel type of acyl-homoserine lactone with a double unsaturated side chain under methylotrophic growth conditions. FEBS Lett. 580(2), 561-567 (2006).
[5]. Schaefer, A.L., Val, D.L., Hanzelka, B.L., et al. Generation of cell-to-cell signals in quorum sensing: Acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protien. Proceedings of the National Academy of Sciences of the United States of America 93, 9505-9509 (1996).
[6]. Welch, M., Todd, D.E., Whitehead, N.A., et al. N-acyl homoserine lactone binding to the CarR receptor determines quorum-sensing specificity in Erwinia. EMBO Journal 19(4), 631-641 (2000).
[7]. Ortori, C.A., Atkinson, S., Chhabra, S.R., et al. Comprehensive profiling of N-acylhomoserine lactones produced by Yersinia pseudotuberculosis using liquid chromatography coupled to hybrid quadrupole-linear ion trap mass spectrometry. Anal. Bioanal. Chem. 387(2), 497-511 (2007).
[8]. Toth, I.K., Newton, J.A., Hyman, L.J., et al. Potato plants genetically modified to produce N-acylhomoserine lactones increase susceptibility to soft rot erwiniae. Molecular Plant-Microbe Interactions 17(8), 880-887 (2004).
[9]. Williams, P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153 (Pt 12), 3923-3938 (2007).
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















