Nifuratel |
| Catalog No.GC10276 |
Products are for research use only. Not for human use. We do not sell to patients.
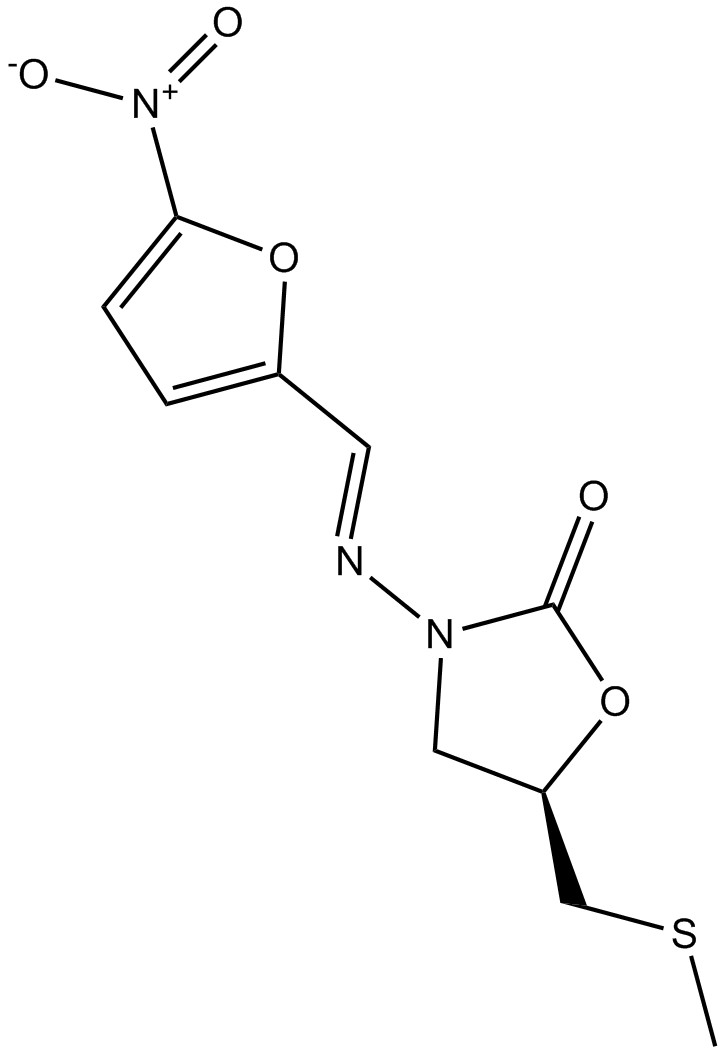
Cas No.: 4936-47-4
Sample solution is provided at 25 µL, 10mM.
Nifuratel(NF 113, SAP 113) is a broad antibacterial spectrum agent, which is used as an antibacterial, antifungal, and antiprotozoal (Trichomonas).IC50 Value: 0.125-1 μg/mL(MIC, A. vaginae) [1]Target: Antibacterial; Antiprotozoal in vitro: In vitro, nifuratel is able to inhibit the growth of A. vaginae, with a MIC range of 0.125-1 μg/mL; it is active against G. vaginalis and does not affect lactobacilli [1].in vivo: Patients were randomized to receive a 2-week course of bismuth subcitrate (8 mg/kg/day, q.d.s.), amoxicillin (50 mg/kg/day, q.d.s.), with either nifuratel (15 mg/kg/day, q.d.s.) or furazolidone (10 mg/kg/day, q.d.s.), plus omeprazole (0.5 mg/kg, once daily) [2].Toxicity: There were no serious adverse reactions and were no withdrawals due to any side-effects. All of side-effects were self-limiting (dark stools, urine discoloration, blackening of the tongue, and others) [3].Clinical trial: N/A
References:
[1]. Polatti F. Bacterial vaginosis, Atopobium vaginae and nifuratel. Curr Clin Pharmacol. 2012 Feb 1;7(1):36-40.
[2]. Nijevitch AA, et al. Helicobacter pylori eradication in childhood after failure of initial treatment: advantage of quadruple therapy withnifuratel to furazolidone. Aliment Pharmacol Ther. 2005 Nov 1;22(9):881-7.
[3]. Nijevitch AA, et al. Nifuratel-containing initial anti-Helicobacter pylori triple therapy in children. Helicobacter. 2007 Apr;12(2):132-5.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















