Phen Green SK diacetate
|
| Catalog No.GC40243 |
Phen Green SK diacetate (PGSK) represents a fluorescent indicator for heavy metals, demonstrating reactivity towards a range of metal ions encompassing Fe2+, Cd2+, Co2+, Ni2+, and Zn2+.
Products are for research use only. Not for human use. We do not sell to patients.
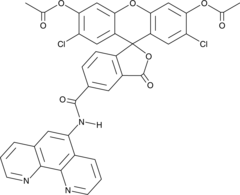
Cas No.: 234075-45-7
Sample solution is provided at 25 µL, 10mM.
Phen Green SK diacetate (PGSK) represents a fluorescent indicator for heavy metals, demonstrating reactivity towards a range of metal ions encompassing Fe2+, Cd2+, Co2+, Ni2+, and Zn2+. PGSK is more sensitive to quenching by the ferrous ion (Fe2+) than by the ferric ion (Fe3+)[1].The excitation/emission peaks of PGSK diacetate are recorded at 507/532 nm, correspondingly, with its fluorescence being suppressed upon interaction with metal ions. Phen Green SK is a membrane-impermeable fluorophore, sensitive only to free (chelatable) iron, which can be loaded into intact cells[2-4]. This compound has found applications in quantifying iron levels in isolated rat hepatocytes, elucidating the metal ion preferences of divalent metal-ion transporter 1 (DMT1), and tracking copper transportation across P. sativum chloroplast membranes and cells[5-7].
References:
[1]. Shingles R, North M, et,al. Direct measurement of ferrous ion transport across membranes using a sensitive fluorometric assay. Anal Biochem. 2001 Sep 1;296(1):106-13. doi: 10.1006/abio.2001.5209. PMID: 11520038.
[2].Illing AC, Shawki A, et,al.Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J Biol Chem. 2012 Aug 31;287(36):30485-96. doi: 10.1074/jbc.M112.364208. Epub 2012 Jun 26. PMID: 22736759; PMCID: PMC3436370.
[3]. Petrat F, de Groot H, et,al. Determination of the chelatable iron pool of single intact cells by laser scanning microscopy. Arch Biochem Biophys. 2000 Apr 1;376(1):74-81. doi: 10.1006/abbi.2000.1711. PMID: 10729192.
[4]. Rauen U, Petrat F, et,al.Hypothermia injury/cold-induced apoptosis--evidence of an increase in chelatable iron causing oxidative injury in spite of low O2-/H2O2 formation. FASEB J. 2000 Oct;14(13):1953-64. doi: 10.1096/fj.00-0071com. PMID: 11023979.
[5]. Petrat F, Rauen U, et,al.Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology. 1999 Apr;29(4):1171-9. doi: 10.1002/hep.510290435. PMID: 10094962.
[6]. Shingles R, Wimmers LE, et,al.Copper transport across pea thylakoid membranes. Plant Physiol. 2004 May;135(1):145-51. doi: 10.1104/pp.103.037895. Epub 2004 Apr 30. PMID: 15122011; PMCID: PMC429342.
[7]. Martins V, Hanana M, et,al. Copper transport and compartmentation in grape cells. Plant Cell Physiol. 2012 Nov;53(11):1866-80. doi: 10.1093/pcp/pcs125. Epub 2012 Sep 5. PMID: 22952251.
Average Rating: 5 (Based on Reviews and 1 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *





