Pimasertib (AS-703026) (Synonyms: MSC1936369B, Pimasertib) |
| Catalog No.GC18050 |
Pimasertib (AS-703026) (AS703026) is a highly selective, ATP non-competitive allosteric orally available MEK1/2 inhibitor.
Products are for research use only. Not for human use. We do not sell to patients.
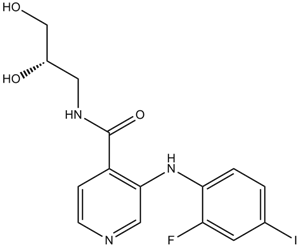
Cas No.: 1236699-92-5
Sample solution is provided at 25 µL, 10mM.
IC50: ranging from 0.005 to 2 μM for the growth of MM cell lines
The MEK/ERK pathway transmits mitogenic signals to the nucleus and thus regulates the expression of genes that are critical for survival, proliferation, and differentiation. This pathway is often upregulated in cancer as result of oncogenic mutations. MEK is a crucial kinase in this pathway and has been targeted for the therapeutic agents development that block the MEK/ERK signaling cascade. AS 703026 is a novel small molecule MEK inhibitor.
In vitro: AS 703026 binds to the distinctive MEK allosteric site and therefore exhibits exquisite kinase selectivity. The AS 703026 binding to MEK is independent of ATP, and results in enzyme inhibition by prevention of activation. This inhibitor shows strong antiproliferative effect on tumor cell lines bearing oncogenic mutations or amplifications along the MEK/ERK pathway [1].
In vivo: In relevant mouse xenograft models, AS 703026 inhibits tumor growth after oral administration. The effect on tumor growth is dose-dependant and correlates with target modulation in both tumors and blood [1].
Clinical trial: 16 patients were enrolled in the trial. 10 and 6 patients were treated daily with 45 and 60 mg of AS 703026 plus FOLFIRI, respectively. The MTD was considered to be 45 mg/day. Of the 15 patients in the efficacy analysis group, 2 patients had partial response, 9 patients had stable disease, 3 patients had progressive disease as their best overall response and 1 patient could not be evaluated.
Reference:
[1] Goutopoulos A, Askew BC, Bankston D, Clark A, Dhanabal M, Dong R, Fischer D, Healey B, Jiang X, Josephson K, Lin J, Ma J, Noonan T, Qiu D, Rocha C, Romanelli A, Shutes A, Spooner E, Tian H, H Y. AS703026: a novel allosteric MEK inhibitor. AACR Annual meeting. 2009 Abstract#4776.
[2] Macarulla T, Cervantes A, Tabernero J et al. Phase I study of FOLFIRI plus pimasertib as second-line treatment for KRAS-mutated metastatic colorectal cancer. Br J Cancer. 2015 Jun 9;112(12):1874-81.
Average Rating: 5 (Based on Reviews and 31 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















