SCH 58261 |
| Catalog No.GC14565 |
A2A adenosine receptor competitive antagonist
Products are for research use only. Not for human use. We do not sell to patients.
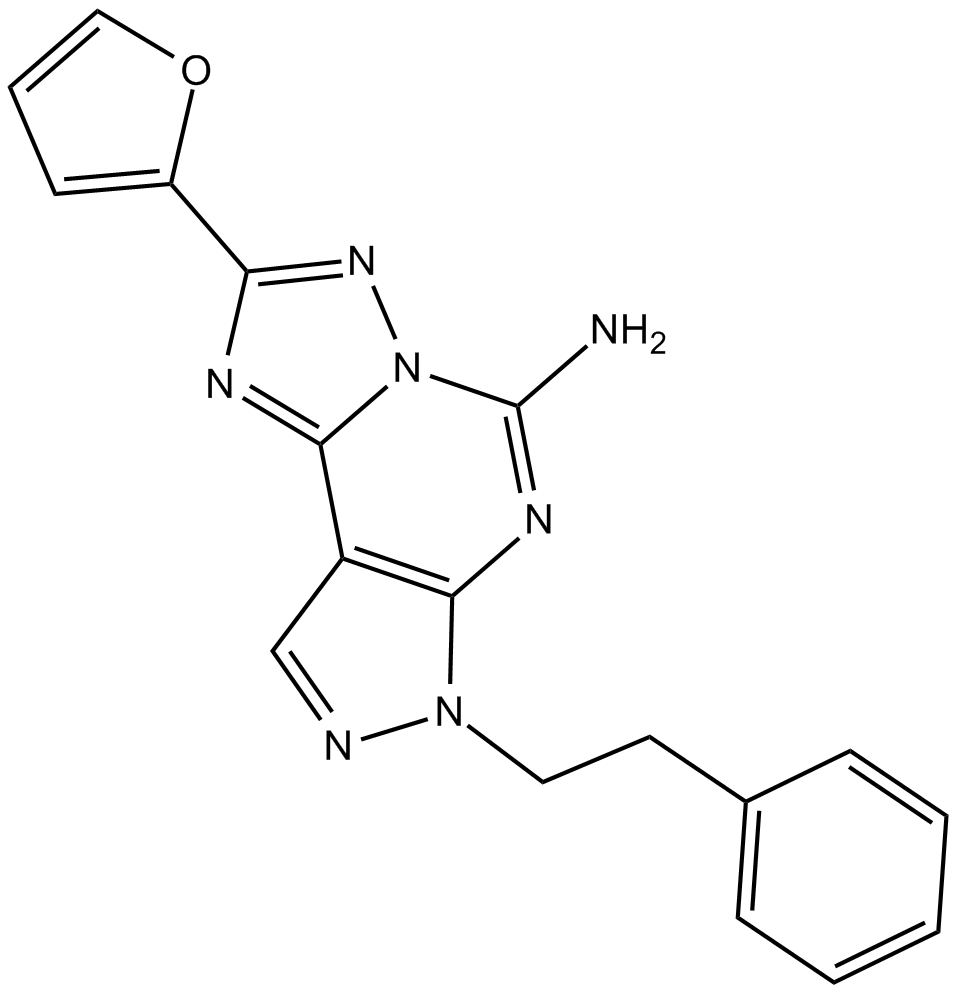
Cas No.: 160098-96-4
Sample solution is provided at 25 µL, 10mM.
SCH 58261 is a potent, selective and competitive antagonist of adenosine A2A receptor with an IC50 of 15 nM, and displays 323-, 53- and 100-fold more selective for A2A receptor than A1, A2B, and A3 receptors, respectively[1][2][3].
SCH 58261 (0 nM–10 µM; 7 days) decreases cell viability in a concentration-dependent in the NSCLC cell line H1975[4].SCH58261 (25 μM; 72 hours) can inhibit the growth of CAF cells[5].
SCH 58261 (2 mg/kg; i.p.; daily; for 20 days) causes a decrease in the tumor burden in a NSCLC mouse model[5].SCH 58261 (5 mg/kg; i.p.; 3 times; every 3 hours; 10 minutes before haloperidol) partially decreases the haloperidol-induced catalepsy and the increase in the PENK mRNA expression in both dorsolateral and ventrolateral parts of the striatum at all three examined levels[6].SCH 58261 diminishes the parkinsonian-like muscle rigidity and potentiates the effect of L-DOPA in rat model[7].
References:
[1]. Zocchi C, et al. Binding of the radioligand [3H]-SCH 58261, a new non-xanthine A2A adenosine receptor antagonist, to rat striatal membranes. Br J Pharmacol. 1996 Apr;117(7):1381-6.
[2]. Varani K, et al. Pharmacological and biochemical characterization of purified A2a adenosine receptors in human platelet membranes by [3H]-CGS 21680 binding. Br J Pharmacol. 1996 Apr;117(8):1693-701.
[3]. Xi J, et al. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009 Nov;47(5):684-90.
[4]. Kuzumaki N, et al. Multiple analyses of G-protein coupled receptor (GPCR) expression in the development of gefitinib-resistance in transforming non-small-cell lung cancer. PLoS One. 2012;7(10):e44368.
[5]. Mediavilla-Varela M, et al. Antagonism of adenosine A2A receptor expressed by lung adenocarcinoma tumor cells and cancer associated fibroblasts inhibits their growth. Cancer Biol Ther. 2013 Sep;14(9):860-8.
[6]. Wardas J, et al. SCH 58261, a selective adenosine A2A receptor antagonist, decreases the haloperidol-enhanced proenkephalin mRNA expression in the rat striatum. Brain Res. 2003 Jul 11;977(2):270-7.
[7]. Wardas J, et al. SCH 58261, an A(2A) adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse. 2001 Aug;41(2):160-71.
Average Rating: 5 (Based on Reviews and 17 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




