SP 600125 |
| Catalog No.GC15344 |
A pan-JNK inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
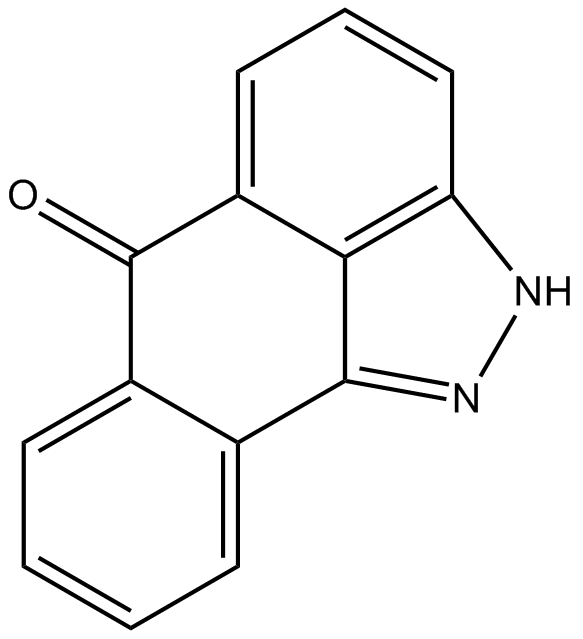
Cas No.: 129-56-6
Sample solution is provided at 25 µL, 10mM.
SP600125 is a selective, reversible and ATP-competitive inhibitor of Jun N-terminal kinase (JNK) with IC50 values of 40, 40 and 90 nM for JNK1, 2 and 3, respectively [1].
SP600125 was screened out from a time-resolved f luorescence assay using the GST-c-Jun and recombinant human JNK2. In this assay, SP600125 showed a Ki value of 190 nM. SP600125 was also found to inhibit JNK1, 2 and 3 isoforms in the selectivity tests. The selectivity of SP600125 for JNK is 300-fold greater than that for ERK1 and p38-2. In Jurkat T cells, SP600125 suppressed the phosphorylation of c-Jun with IC50 of 5-10 μM. SP600125 also inhibited the expression of IL-2 and IFN-γ in cells stimulated with PMA and phytohemagglutinin, since JNK had been reported to regulate the transcription of IL-2. Besides that, SP600125 exerted differential inhibition of cytokines in CD4+ cells as well as inflammatory genes in monocytes. Moreover, SP600125 administration significantly inhibited TNF-α expression induced by LPS in a mouse model, suggesting that it had efficacy in endotoxin-induced inf lammation in vivo [1].
References:
[1] Bennett B L, Sasaki D T, Murray B W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proceedings of the National Academy of Sciences, 2001, 98(24): 13681-13686.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




