Triflusal (Synonyms: UR 1501) |
| Catalog No.GC13300 |
COX inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
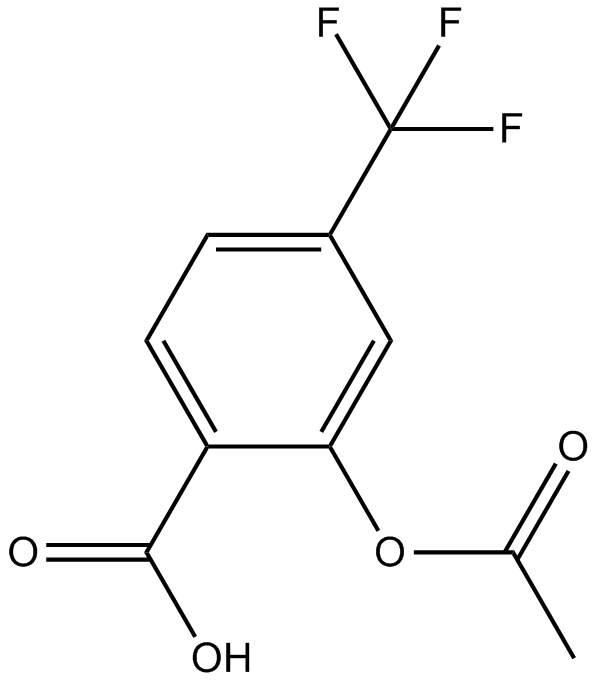
Cas No.: 322-79-2
Sample solution is provided at 25 µL, 10mM.
Triflusal irreversibly inhibits the production of thromboxane-B2 in platelets by acetylating cycloxygenase-1.Target: COXTriflusal at 10 mM, 100 mM and 1 M decreases LDH efflux in rat brain slices after anoxia/reoxygenation by 24%, 35% and 49% respectively. Triflusal also reduces inducible NO synthase activity by 18%, 21% and 30% [1].Triflusal (10 mg/kg i.v.) reduces platelet deposition on subendothelium-induced primary thrombus by about 68% in rabbits. Triflusal (10 mg/kg i.v.) reduces platelet deposition on a fresh thrombus formed over tunica media by about 48% in rabbits. Triflusal (40 mg/kg p.o.) reduces platelet deposition on a primary thrombus triggered by subendothelium and tunica media by 53% in rabbits. Triflusal (40 mg/kg p.o.) significantly reduces Cox-2 mRNA levels and protein levels without influence Cox-1 mRNA levels on the vascular wall in rabbits [2]. Triflusal (600 mg/day for 5 days) results in an increase in NO production by neutrophils and an increase in endothelial nitric oxide synthase (eNOS) protein expression in neutrophils in healthy volunteers [3].
References:
[1]. Fernández de Arriba A, et al. Inhibition of cyclooxygenase-2 expression by 4-trifluoromethyl derivatives of salicylate, triflusal, and its deacetylated metabolite, 2-hydroxy-4-trifluoromethylbenzoic acid. Mol Pharmacol. 1999 Apr;55(4):753-60.
[2]. Duran, X., et al., Protective effects of triflusal on secondary thrombus growth and vascular cyclooxygenase-2. J Thromb Haemost, 2008. 6(8): p. 1385-92.
[3]. De Miguel, L.S., et al., A 4-trifluoromethyl derivative of salicylate, triflusal, stimulates nitric oxide production by human neutrophils: role in platelet function. Eur J Clin Invest, 2000. 30(9): p. 811-7.
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















