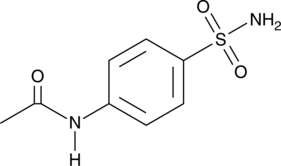
4-Acetamidobenzenesulfonamide (Synonyms: APAS, N-Acetyl p-Aminobenzene Sulfonamide, N4-Acetyl Sulfanilamide, N-Acetylsulfanilamide, NSC 217, NSC 406839) |
| Catalog No.GC49337 |
A metabolite of asulam and sulfanilamide
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 121-61-9
Sample solution is provided at 25 µL, 10mM.
4-Acetamidobenzenesulfonamide is a metabolite of the herbicide asulam and the sulfonamide antibiotic sulfanilamide .1,2,3 It is formed from asulam by conversion to sulfanilamide via intestinal microflora, followed by N4-acetylation in the liver. 4-Acetamidobenzenesulfonamide inhibits carbonic anhydrase II (CAII), CAIX, and CAXII (Kis = 246, 135, and 49 nM, respectively, for the human enzymes).4 It has also been used in the synthesis of compounds with antibacterial activity.5
1.Heijbroek, W.M., Muggleton, D.F., and Parke, D.V.Metabolism of the carbamate herbicide, asulam, in the ratXenobiotica14(3)235-247(1984) 2.Okumura, F., Ueda, O., Kitamura, S., et al.N-acetylation and N-formylation of carcinogenic arylamines and related compounds in dogsCarcinogenesis16(1)71-76(1995) 3.Olsen, H., and MØrland, J.Sulfonamide acetylation in isolated rat liver cellsActa Pharmacol. Toxicol. (Copenh)49(2)102-109(1981) 4.Ozensoy, O., Puccetti, L., Fasolis, G., et al.Carbonic anhydrase inhibitors: Inhibition of the tumor-associated isozymes IX and XII with a library of aromatic and heteroaromatic sulfonamidesBioorg. Med. Chem. Lett.15(21)4862-4866(2005) 5.Wang, X.-L., Wan, K., and Zhou, C.-H.Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activitiesEur. J. Med. Chem.45(10)4631-4639(2010)
Average Rating: 5 (Based on Reviews and 20 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















