AMI-1 |
| Catalog No.GC17275 |
A cell permeable inhibitor of PRMTs
Products are for research use only. Not for human use. We do not sell to patients.
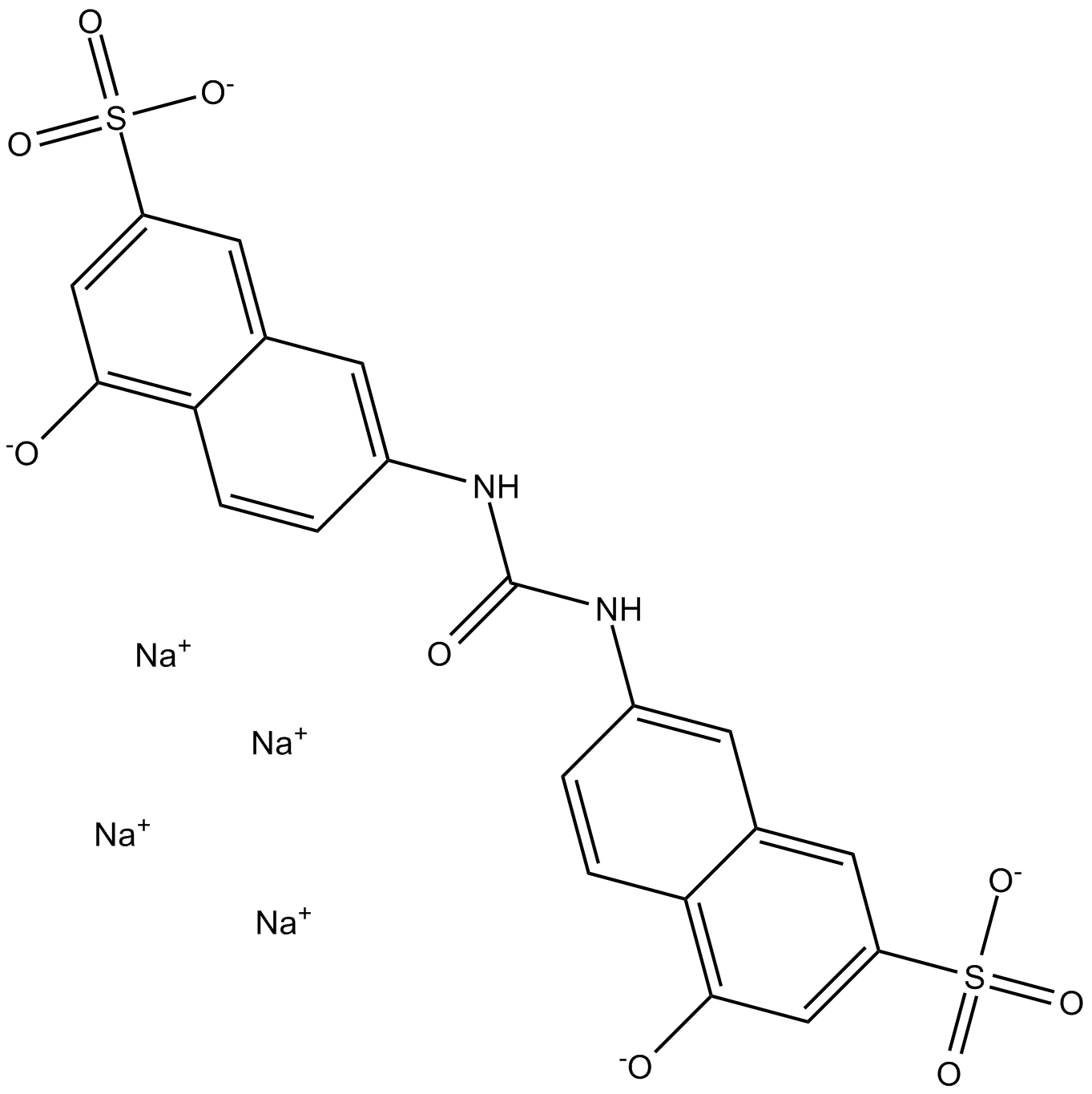
Cas No.: 20324-87-2
Sample solution is provided at 25 µL, 10mM.
AMI-1 is a potent, cell-permeable compound which inhibits protein arginine N-methyltransferases (PRMTs), including human PRMT1 (IC50 = 8.8μM) and yeast-Hmt1p (IC50 = 3.0μM), by blocking peptide-substrate binding.IC50 value: 8.8μM (human PRMT1), 3.0μM (yeast-Hmt1p)Target: human PRMT1, yeast-Hmt1pin vitro: AMI-1 suppresses the transcriptional coactivator activity of PRMT1 and PRMT4 and it inhibits HIV-1 RT polymerase (IC50 = 5.0μM). PRMT1 methylates histone H4, and is essential for other subsequent histone modifications.[1] AMI-1 is the most active nonpeptidic inhibitor reported to be selective against PRMT1. AMI-1 is a selective PRMT inhibitor with a bisanionic structure that is related to compounds known to generate pleiotropic interactions with many proteins, should be further optimized before exploring additional binding pockets. [2]in vivo: AMI-1 is administered intranasally to chronic AIPI rats to determine PRMT effects on asthmatic parameters. AMI-1 inhibited the expression of COX2 in TGF-β-stimulated cells. AMI-1 administered to AIPI rats reduced COX2 production and humoral immune response, and it abrogated mucus secretion and collagen generation.[1]
References:
[1]. Sun Q, et al. PRMT1 Upregulated by Epithelial Proinflammatory Cytokines Participates in COX2 Expression in Fibroblasts and Chronic Antigen-Induced Pulmonary Inflammation. J Immunol. 2015 Jul 1;195(1):298-306.
[2]. Castellano S, et al. Design, synthesis and biological evaluation of carboxy analogues of arginine methyltransferase inhibitor 1 (AMI-1). ChemMedChem. 2010 Mar 1;5(3):398-414.
[3]. Lv L, et al. PRMT1 promotes glucose toxicity-induced β cell dysfunction by regulating the nucleo-cytoplasmic trafficking of PDX-1 in a FOXO1-dependent manner in INS-1 cells. Endocrine. 2015 Aug;49(3):669-682.
[4]. Wang J, et al. Pharmacophore-based virtual screening and biological evaluation of small molecule inhibitors for protein arginine methylation. J Med Chem. 2012 Sep 27;55(18):7978-7987.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















