Amonafide (Synonyms: AS1413, Benzisoquinolinedione, Nafidimide, NCI 308847, NSC 308847) |
| Catalog No.GC11019 |
DNA intercalator,Topo II inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
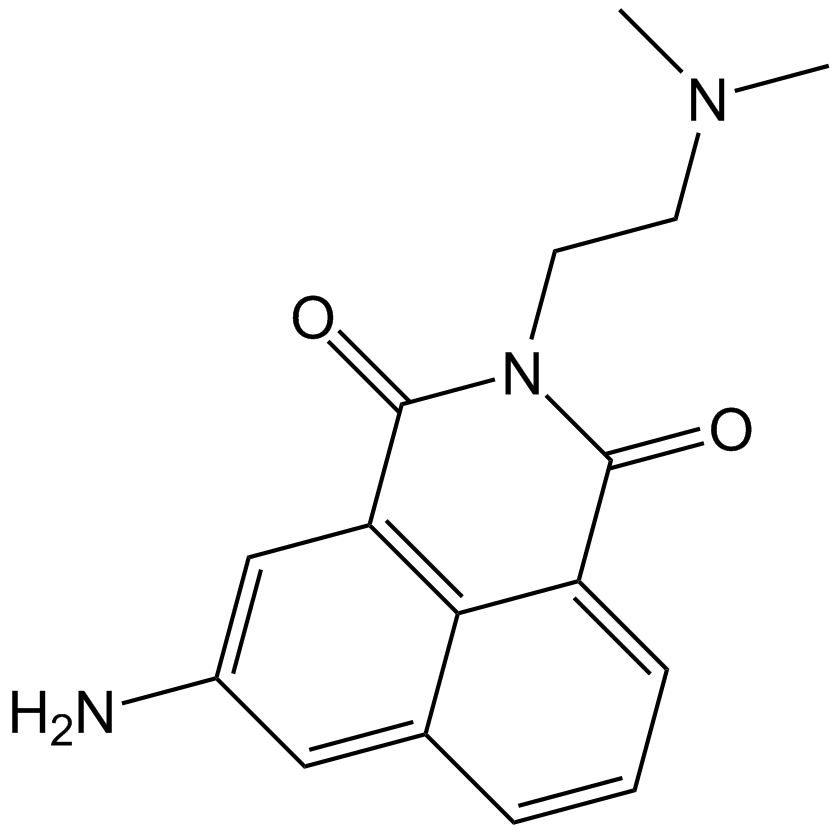
Cas No.: 69408-81-7
Sample solution is provided at 25 µL, 10mM.
IC50: 4.67, 2.73, and 6.38 for HT-29, HeLa, and PC3 cells, respectively
Amonafide is a novel topoisomerase II inhibitor. Topoisomerase II plays critical roles including DNA transcription, replication and chromosome segregation. Though the biological functions of topoisomerase II are important for insuring genomic integrity, the ability to interfere with topoisomerase II and generate enzyme mediated DNA damage is an effective strategy for cancer chemotherapy.
In vitro: Amonafide intercalated with DNA and disrupted the loading of topoisomerases. In contrast to the classic agents, amonafide was found to induce higher molecular weight fragmentation, resulting in the apoptosis without DNA cleavage. Amonafide was found to act in an ATP-independent manner and seemed unlikely to induce the chromosome translocations associated with treatmentinduced leukemia [1].
In vivo: Amonafide was found to be able to inhibit IP L1210 leukemia, with optimal increased life spans (ILS) of 61% to 106% following single 16 mg/kg dosing on days 1 to 9. Similar efficacy was noted against IP P388 murine leukemia and the SC implanted L1210 leukemia. Additionally, amonafide demonstrated activity against two nonleukemic IP implanted murine tumors, the M5076 sarcoma and the B16 melanoma [2].
Clinical trial: A multicenter, open-label, combination Phase II study was conducted for patients with sAML, evaluating the efficacy of cytarabine and amonafide administered as a continuous iv. Infusion of cytarabine on days 1-7 combined with 600 mg/m2/day for days 1-5[1].
References:
[1] Freeman CL,Swords R,Giles FJ. Amonafide: a future in treatment of resistant and secondary acute myeloid leukemia Expert Rev Hematol.2012 Feb;5(1):17-26.
[2] Saez R,Craig JB,Kuhn JG,Weiss GR,Koeller J,Phillips J,Havlin K,Harman G,Hardy J,Melink TJ, et al. Phase I clinical investigation of amonafide. J Clin Oncol.1989 Sep;7(9):1351-8.
Average Rating: 5 (Based on Reviews and 1 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















