Avibactam (Synonyms: NXL-104 free acid) |
| Catalog No.GC14602 |
A β-lactamase inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
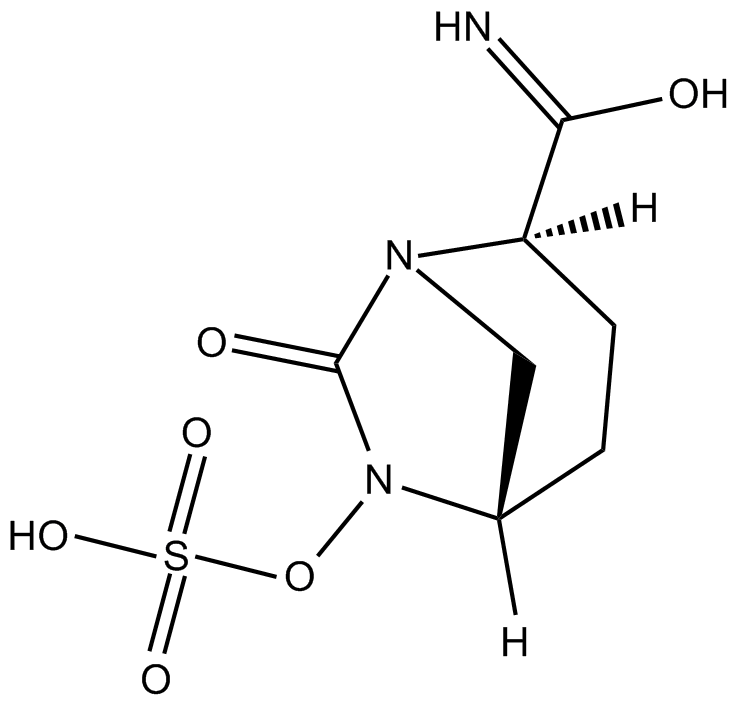
Cas No.: 1192500-31-4
Sample solution is provided at 25 µL, 10mM.
Avibactam free acid (NXL-104 free acid) is a covalent, reversible non-β-lactam β-lactamase inhibitor, inhibits β-lactamase TEM-1 and CTX-M-15 with IC50 of 8 nM and 5 nM, respectively.
Avibactam (NXL104) is a molecule with little antibacterial activity, that inhibits class A and C β-lactamases. Avibactam (NXL104) inactivates most important β-lactamases except metallo types and Acinetobacter OXA carbapenemases[2].
Avibactam (NXL104) sodium displays a slow return of activity with an off-rate of 0.045±0.022 min-1, which converts to a residence time half-life (tt1/2) of 16±8 min. The measured off-rate for Avibactam (NXL104) suggests that slow deacylation through hydrolysis or reversibility is occurring, and it is in contrast to previously reported extremely long t1/2 values of >1 or >7 d for Avibactam (NXL104) inhibition of TEM-1[1]. Avibactam is a new promising β-lactamase inhibitor, to overcome resistance caused by β-lactamases. Mice are infected with ca.106 CFU of Pseudomonas aeruginosa intramuscularly into the thigh or intranasally to cause pneumonia and are given 8 different (single) subcutaneous doses of Ceftazidime and Avibactam (NXL104) in various combined concentrations, ranging from 1 to 128 mg/kg of body weight in 2-fold increases. The mean estimated half-life in plasma of Ceftazidime in the terminal phase is 0.28 h (SD, 0.02 h), and that of Avibactam is 0.24 h (SD, 0.04 h). Volumes of distribution are 0.80 liters/kg (SD, 0.14 liters/kg) and 1.18 liters/kg (SD, 0.34 liters/kg), respectively[3].
References:
[1]. Ehmann DE, et al. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A. 2012 Jul 17;109(29):11663-8.
[2]. Livermore DM, et al. Characterization of β-lactamase and porin mutants of Enterobacteriaceae selected with ceftaroline + avibactam (NXL104). J Antimicrob Chemother. 2012 Jun;67(6):1354-8.
[3]. Berkhout J, et al. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother. 2015 Apr;59(4):2299-304.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




