Cenicriviroc
Cenicriviroc, shortened as CVC is a novel and small compound of potential clinical significance. Other names of Cenicriviroc compound used in the scientific literature include TAK-652, TBR-652. As it was first discovered from the chemical manipulation of TAK-779 compound; it is quite obvious that it is a chemical derivative of the same compound, specifically it is a sulfoxide derivative of TAK-779 compound. Ease of synthesis was the primary stimulus for the further research on this compound.
A two-dimensional view of the Molecular Structure of the Cenicriviroc compound is given in Figure 1. Its high binding potency with the CCR5 is due to the presence of [6,8]- fused rings in its molecular structure.
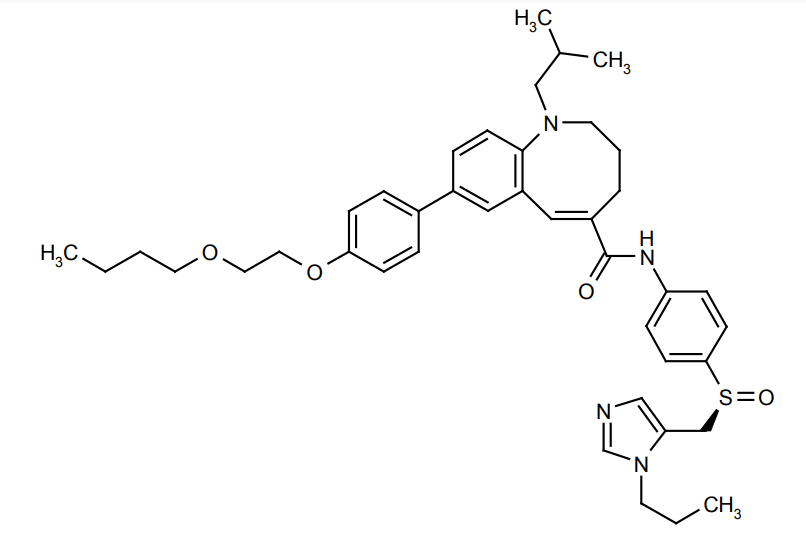
Figure 1 A two-dimensional view of the Molecular Structure of the Cenicriviroc compound. Polar sulfoxide moiety replaces quaternary ammonium; [6,8]-fused nuclei substitutes [6,7]-fused nuclei; 4-(2-butoxyethoxy) phenyl group substitutes 4-methylphenyl group and 1-propylimidazolyl group replaces pyranyl group in the molecular structure of the Cenicriviroc compound when compared to the molecular structure of its parent compound, i.e., TAK-779 compound.
The Cenicriviroc compound effectively binds to CCR5 and inhibits it; thus, possess and antiretroviral effect as CCR5 has an assistive role in the entry of HIV-I into the host cell.
As Cenicriviroc compound not only binds and inhibit CCR5 but also does the same with CCR2. This nature of Cenicriviroc is called dual inhibitor which is exploited in the research for the potential treatment of the liver fibrosis in the Phase 2b of clinical trials; a medical condition in which CCR2 along with the CCR5 initiates the inflammatory signalling and also the immune cell infiltration and contributes to liver fibrosis.
CCR2 and CCR5 stand for C-C Chemokine Receptor 2 and C-C Chemokine Receptor 5, respectively. Both CCR2 and CCR5 are C-C-chemokine receptors (CCRs), which are a type of receptors that are commonly expressed receptors on the cells of the immune system and involved in a number of vital immune processes, most importantly in migration of white blood cells, which are also known as leukocytes; interestingly C-C Chemokine Receptor 5 (CCR5) also gets commonly expressed in the microglia, i.e., microglia comprise of the immune cells that especially protect the brain, besides that it is also expressed in the neuron and the astrocytes but in extremely lesser amount. Moreover, C-C Chemokine Receptor 5 (CCR5) is majorly present in the hippocampal CA1 that is a region in human brain that is responsible for the memory consolidation, i.e., episodic memory as well as the spatial memory. The spatial memory is helpful in the navigation process. Both of them belong to a protein superfamily, i.e., G-protein coupled receptors (GPCRs). Being GPCRs, it is quite obvious that both are composed of a transmembrane embedded in the plasma membrane and having seven turns as the most prominent and a distinguishing structural feature.
CCR5 as it is a receptor protein, i.e., a protein that has the inherent ability to initiate one or multiple signalling pathways within the biological cell upon interacting and binding to its legitimate ligand, is expressed in the T-cells and in those macrophages only which are expressing CD4 and after being expressed it gets localized as a surface protein on the plasma membrane of these cell. For CCR5, Macrophage Inflammatory Proteins (MIPs) specifically their names are MIP-1α and MIP-1β along with the Regulated upon Activation, Normal T Cell Expressed and Secreted shortened as RANTES are the legitimate ligands, thus CCR5 can be more appropriately called as coreceptor rather than simply a receptor. Besides this, clinically the more important functionality of CCR5 is that it facilities the entry of viruses, specifically HIV-1, in the human body.
The entry of the HIV-I virus in the host cell is mediated by a molecular complex, specifically a glycoprotein complex (gp) lying on the envelop of the HIV-I virus. This glycoprotein complex (gp) is encoded by the gene namely Env. The complex is composed of two types subunits which are non-covalently bounded to each other. One subunit lies on the surface of virus as a gp120 surface subunit while other one is embedded in the viral membrane as a gp41 transmembrane subunit. Overall, the glycoprotein complex is composed of three subunits of each type in the form of two trimers. The underlying mechanism how this glycoprotein complex is helping the HIV-I virus to get entry to the host cell involves the binding of the gp120 surface trimer subunit with the CD4+ receptor. This first interaction induces some conformational changes within the gp120 subunit that enables it to bind with the CCR5. In addition to your this, conformational changes within the surface gp120 subunit also assist in the process of inserting gp41 fusion peptide segment into the plasma membrane of the host cell; this ultimately leads towards the fusion of HIV-I virus with the host cell and initiation of viral infection which is marked by the replication of HIV-I virus. This first interaction also leads to the formation of CD4/gp120 complex, a molecular complex that is essential for the interaction between the viral envelope and the CCR5. The CD4/gp120 complex interacts and binds with the CCR5 through the specific V3 region, a highly specific sequence of amino acids. In binding to the CCR5, N-terminal domain as well as the second extracellular loop (ECL) are involved. All these interactions are clearly presented in Figure 2.
Thus, it is considered as an important therapeutic target for the treatment of HIV-I. The interactions between CD4/gp120 complex and the CCR5 are effectively inhibited by the introduction of Cenicriviroc when the Cenicriviroc compound blocks the binding site of the CCR5. Cenicriviroc does so at the IC50 of 0.10 nM which is the minimum concentration/ amount when compared to other inhibitors. Moreover, [6,8]-fused 1-benzazocine acts as nuclei providing an optimal core for the Env mediated membrane fusion of HIV-I. Furthermore, along with this fused rings, 1-isobutyl and 1-propyl-1H-imidazol-5-yl both are such structural features that are essential to the anti-HIV activity. As Cenicriviroc was the only candidate showing these structural features; thus, it gets selected for further trials and research.
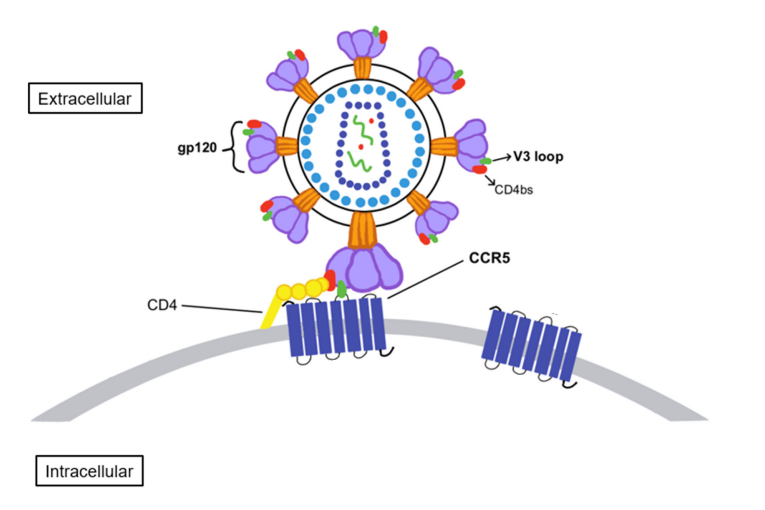
Figure 2 The interaction between the gp120 subunit of the glycoprotein complex (gp) with the CCR5 through the V region, that ultimately facilities the entry of HIV virus into the host cell
There are some neural pathologies that are associated with the HIV infection. CCR5 reduces the neural plasticity in the cortical as well as in the hippocampal regions of the human brain. On the molecular level, upon the binding between the gp120 subunit of the glycoprotein complex (gp) with the CCR5 two distinct signalling pathways are activated; one is acute while other is chronic. Both of these signalling pathways are schematically indicated in Figure 3. In acute pathway, microglia are abnormally activated and leads to neuroinflammation, in chronic pathway, CREB, MAPK and DLK are abnormally inhibited and leads to reduced synaptic plasticity. Both these pathways collectively lead to the reduced neural plasticity and cognitive abilities.
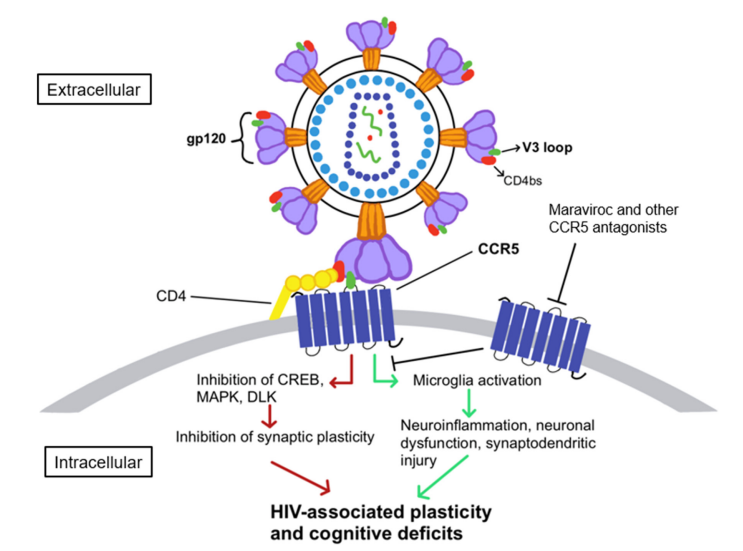
Figure 3 The downstream signaling of CCR5 that leads to the reduction in the neural plasticity and the cognitive abilities.














Comments