JNJ 63533054
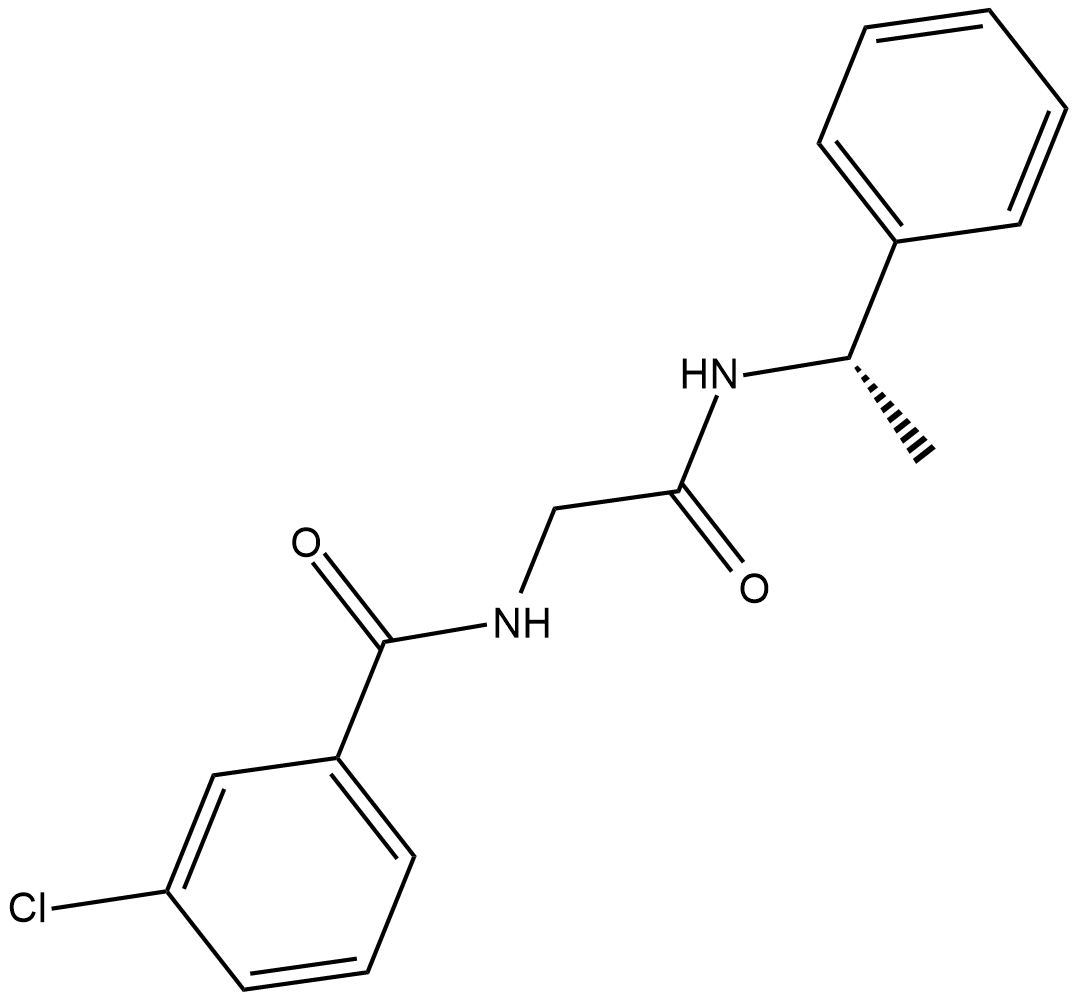
S-3-chloro-N-(2-oxo-2-((1-phenylethyl) amino) ethyl) benzamide is the chemical name of JNJ 63533054. JNJ 63533054 is a small novel molecule. The complete process of its synthesis and identification in the laboratory, is well documented in the scientific literature. Its two dimensional molecular structure is diagrammatically depicted in Figure 1.
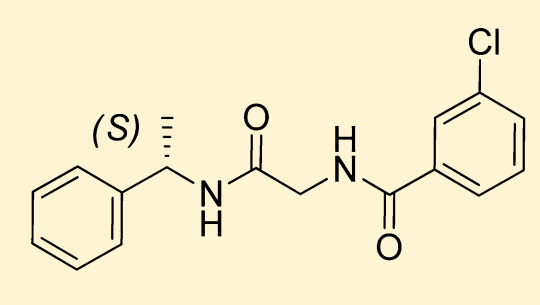
Figure 1 The Molecular Structure of JNJ 63533054
JNJ 63533054 effectively and selectively interacts with and activates the GPR139 which is also called as GPRg1 or GPCR12. Therefore, it is regarded as an agonist for GPR139. GPR139 is a G-protein coupled receptor, that implicitly means that is composed of seven domains which are completely embedded in the lipid bilayer structure of the plasma membrane, thus termed as “transmembrane” domains. GPR139 is a G-protein coupled receptor that needs a ligand to get activated, thus it is regarded as an orphan G-protein coupled receptor. It is a novel GPCR protein. The G protein could be either miniGs/q or Gi in either GTP-bound or GDP-bound status as graphically represented in Figure 2 via the experimental results of the Bioluminescence resonance energy transfer BRET Analysis; therefore, GPR139 could form distinct complexes structures with the same kind of ligand molecule. The structures of these different molecular complexes are depicted in Figure 3, Figure 4, Figure 5 and Figure 6. And, a zoomed in view of the interactions present within these complexes along with the GDP and the GTP nucleotides are diagrammatically shown in Figure 7.
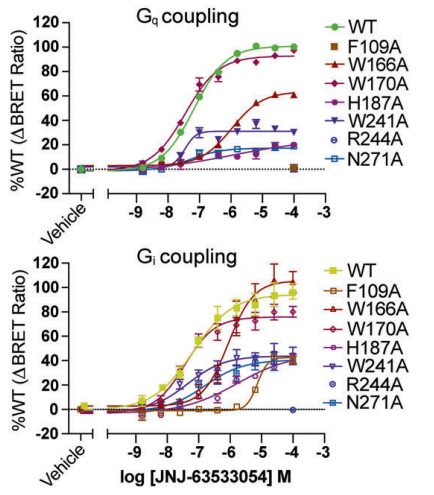
Figure 2 The validation of the involvement of either of the G protein subtype, Gs or Gi in the binding of JNJ 63533054 ligand molecules with the GPR139 via the results of the Bioluminescence resonance energy transfer BRET Analysis
Under normal physiological conditions, GPR139 is mainly expressed in some regions of the Central Nervous System, i.e., different parts of brain namely medial habenula, lateral caudate nucleus, and hypothalamus, thalamus, pituitary gland, locus coeruleus, dorsal root ganglia, ventral tegmental area, NAc, dorsal striatum. These brain regions control the levels of the motivation for the hard work. Furthermore, it has been observed in the mice models in which GPR139 gene was lacking, that all these mice show an impaired phenotypical behaviour towards being a doer that is consistently observed along with the gustatory reward. A pathological condition that is named as apathy which is primarily psychologic in nature and is marked by the lack of motivation to do work for the sake of any reward. As Apathy is a common symptom of different neurological diseases namely, depression, schizophrenia, Alzheimer's disease and Parkinson's disease. Therefore, directly targeting GPR139 in those brain regions is the proposed therapeutical treatment for the decrease in the levels of Apathy in all of these neurological conditions, in a clinical setting. These studies are not performed with our chemical of interest, which is JNJ 63533054, rather than another agonist of GPR139. The roles of GPR139 are poorly understood. Although, it has been also found that these regions also control the movements in the mice model. Furthermore, schizophrenia-like symptoms were present in this mice model lacking in GPR139, for example, late onset hyperactivity, increased stereotypy, increased anxiety-related behavioural traits, impaired acquisition of operant responsiveness, very bad cued fear conditioning and lack of social interaction. These behavioural and neurological symptoms were effectively treated by giving those mice a treatment with GPR139 agonist; in this research although again, JNJ 63533054 was not used to estimate the clinically significant roles of GPR139 agonist.
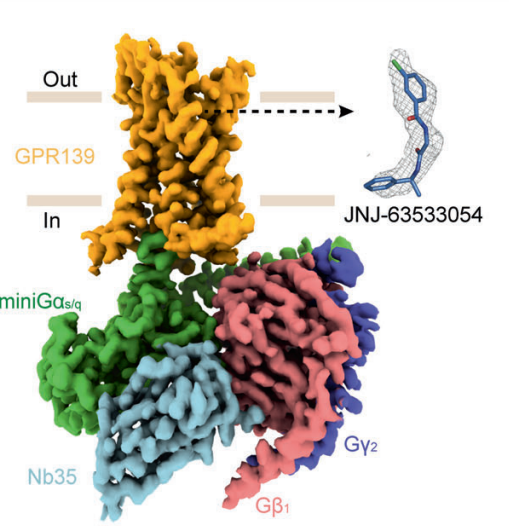
Figure 3 The density map of GPR139-miniGs/q complex created via cryo-Electron Microscopy, in which GPR139 is ready to accept the ligand, i.e., JNJ 63533054.
JNJ 63533054 possess ideal drug properties; this indicates that JNJ 63533054 could be a potential future drug as the underlying mechanism for many of the approved drugs is the targeting of G-protein coupled receptors GPCRs. These properties include high chemical stability in the microsomes from human and rats, high solubility in the water-based solvent, high permeability in the cell along with no efflux which means that the JNJ 63533054 molecules rigorously enter the cells and once entered these molecules can not leave those cells. The last property was especially important and critical as the drug target, which is GPR139, resides within the Central Nervous system CNS.
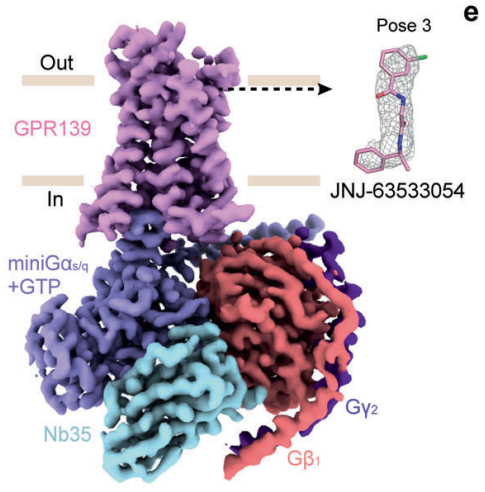
Figure 4 The density map of GTP-bound GPR139–JNJ-63533054–miniGs/q–Nb35 complex created via cryo-Electron Microscopy, in which GPR139 is ready to accept the ligand, i.e., JNJ 63533054 in a distinct molecular orientation. This different molecular orientation is called pose 3 in this figure
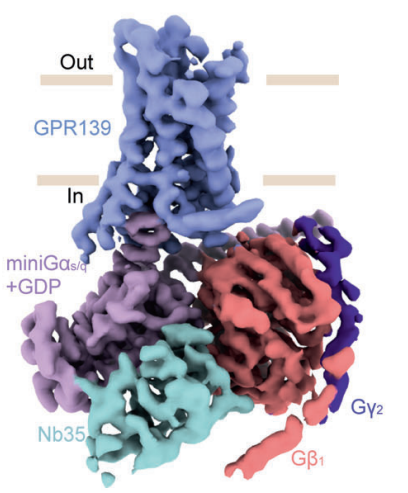
Figure 5 The density map of GDP-bound GPR139–JNJ-63533054–miniGs/q–Nb35 complex, this complex cannot take the ligand, i.e., JNJ 63533054
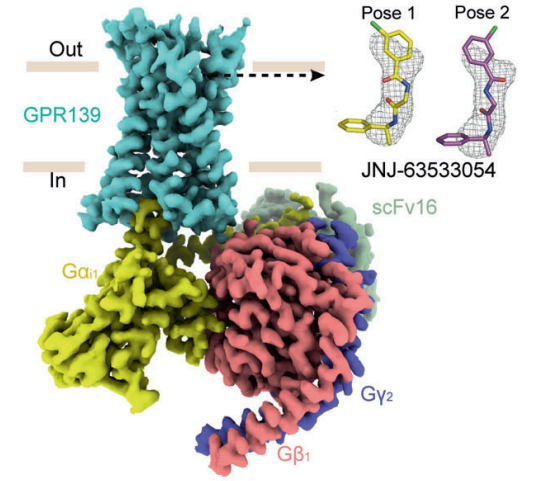
Figure 6 The density map complex created via cryo-Electron Microscopy, in which GPR139 is ready to accept the ligand, i.e., JNJ 63533054 in either of the molecular orientations. These possible molecular orientations are called as pose 1 and pose 2 JNJ 63533054.
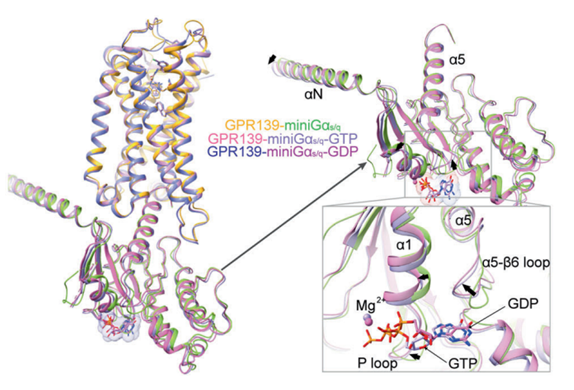
Figure 7 A zoomed in image of the interactions between the GPR139, the G protein along with the GDT and GTP nucleotides
GPR139 protein is encoded by GPR139 gene. The complete GPR139 gene comprises of two exons, and is located on the chromosome number 16 in the human genome. The GPR139 gene is also previously named as GPCR12, PGR3, KOR3L and GPRg1 in the scientific literature. The GPR139 protein gets activated upon binding with any of its endogenous ligands or any exogenous ligand, i.e., in this case the ligand is JNJ 63533054. JNJ 63533054 binds in the same pocket that is formed by the bunch of seven helices present in the transmembrane domains; irrespective of the chemical nature of the G protein coupled, thus this binding is a common feature of both possible complexes. Once activated, the GPR139 protein performs the neuromodulation via the progression of the neuromodulatory signals, that means that it affects the electrical activity of the nerve cells/ tissues in terms of neuron excitation, neuron firing and establishment of the synapses, according to the specific stimuli in the external environment. Through neuromodulation, the neural networks changes, i.e., being plastic, and thus all types of the behavioural responses, namely cognition, emotions, social interactions and eating patterns, are changed as they directly influence the integration of cognition and neuromotor. Any changes to these neuromodulatory signals can possibly lead to several neuropsychiatric conditions. It does so by activating numerous G protein signalling pathways, particularly Gq and Gi1 signalling pathways as schematically demonstrated in Figure 8. On the cell level, release of Calcium ions, phosphorylation of ERK and the modulation of cyclic AMP are the major cellular responses to the activated GPR139. Within a cell, GPR139 is expressed along with dopamine D2 receptors (D2R) and also affects the signalling that is mediated via dopamine D2 receptors (D2R). Moreover, GPR139 also interacts with μ-opioid system and thus affect the neuron activity.
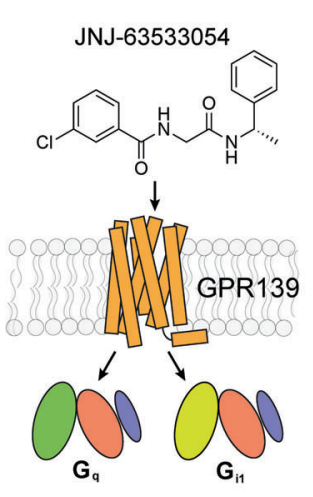
Figure 8 The Activation of downstream Signaling Pathways namely Gq and Gi1, upon the activation of the GPR139 protein via the binding with its ligand, i.e., JNJ 63533054













Comments