Mdivi 1
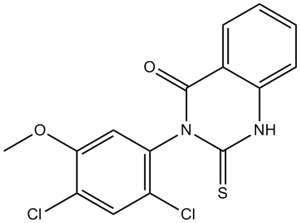
Mitochondrial division inhibitor-1 (Mdivi-1) ( mol.weight : 353.22, empirical formula: C15H10Cl2N2O2S) is a small chemical inhibitor of mitochondrial division dynamin, which plays multiple roles in mitochondrial dynamics, mitochondrial autophagy, ATP production, the immune response, and Ca²⁺ homeostasis.
Mdivi‑1 could be a inhibitor of the mitochondrial division protein dynamin‑related protein 1 (Drp1). Mdivi‑1 was included to H9c2 cells for 3 h, after which, the cells were treated with sodium azide for 24 h. Cell reasonability was measured by Cell Counting Kit-8 measure. pre-treatments with Mdivi‑1 weakened sodium azide‑induced H9c2 cell passing. Mdivi‑1 pre-treatments too repressed the sodium azide‑induced downregulation of Bcl‑2 expression and upregulation of Bax and Drp1 expression. the component of sodium azide‑induced cell passing in H9c2 cells may include the mitochondria‑dependent apoptotic pathway. The Mdivi‑1 may have a cardioprotective impact against sodium azide‑induced apoptosis in H9c2 cardiac muscle cells.
Mitochondrial combination and division play introductory corridor inside the control of apoptosis. Mitochondrial combination proteins prostrate apoptosis by ruining release of cytochrome c from mitochondria, in parcel by controlling cristae structures. Mitochondrial division progresses apoptosis by an pall instrument. Mdivi- 1( for mitochondrial division asset) chokes mitochondrial division in incentive and mammalian cells by particularly bridling the mitochondrial division energetic. In cells, mdivi- 1 ruins apoptosis by precluding mitochondrial outside film permeabilization. In vitro, mdivi- 1 skillfully places shot- actuated Bax/ Bak-dependent cytochrome c release from mitochondria. The mitochondrial division energetic easily coordinates mitochondrial outside film permeabilization independent of Drp1- intermediated division. Mdivi- 1 talks to a new assignment of rectifiers for stroke, a myocardial dead towel, and neurodegenerative conditions.
Tumor corruption factor-related apoptosis-inducing ligand (Path) based procedure could be a focused on helpful approach for the treatment of a assortment of cancers counting ovarian cancer. mitochondrial division inhibitor-1 (mdivi-1) is able to improve the affectability of human ovarian cancer cells to passing receptor ligands counting Path, FAS ligands, and TNF-α. Imperatively, the combination of Path and mdivi-1 has no clear cytotoxic impact on non-transformed human cells, showing a critical helpful window. The upgraded adequacy of combination of mdivi-1 and passing ligands isn't dependent on the initially detailed target of mdivi-1, Drp1, and is additionally not subordinate on the two critical pro-apoptotic Bcl-2 family proteins Bax and Bak.
Mdivi‑1 could be a inhibitor of the mitochondrial division protein dynamin‑related protein 1 (Drp1). The nearness of a profoundly preserved GTPase space in Drp1 shows that Drp1 is included in fundamental capacities of mitochondria and cell. The dynamin proteins have a huge GTPase space comprising of ~300 buildups. The four spaces are the N-terminal GTPase space, center space, variable or B space, and the C-terminal GTPase effector space.
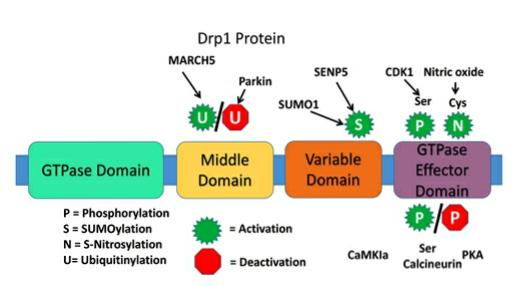
Figure 1: Structure of dynamin-related protein 1.
In skeletal muscle filaments, mitochondria are thickly stuffed adjoining to myofibrils since adenosine triphosphate (ATP) is required to fuel sarcomere shortening. The impacts of Mitochondrial Division Inhibitor 1 (mdivi-1), an inhibitor of mitochondrial parting, on the structure and work of both mitochondria and myofibrils in skeletal muscle tissues built on micromolded gelatin hydrogels was analyzed. Treatment with mdivi-1 did not modify myotube morphology, but did increment the mitochondrial turbidity and oxidative capacity, steady with diminished mitochondrial parting. At last, mdivi-1 expanded sarcomere length, possibly due to mdivi-1-induced changes in mitochondrial volume and compression of myofibrils. Together, these comes about recommend that mdivi-1 increments contractile push era, which may be caused by an increment in maximal breath and/or sarcomere length due to expanded volume of person mitochondria.
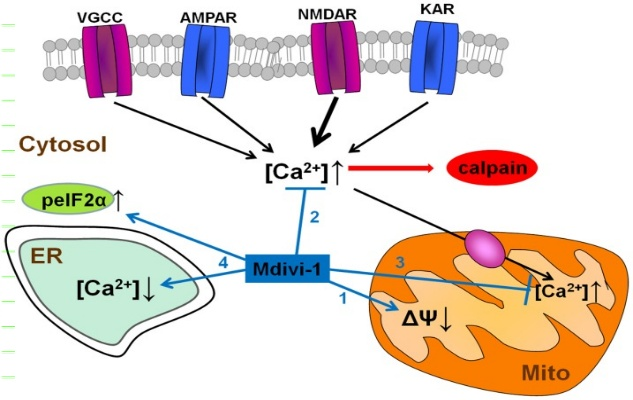
Figure 2: Mdivi-1 actuates a direct hindrance of mitochondrial breath, and a drop in mitochondrial layer potential (1). It moreover weakens cytosolic Ca2+ over-burden due to actuation of VGCCs and glutamate ionotropic receptors, and thus, downstream NMDAR-dependent calpain enactment (2). Mdivi-1-induced mitochondrial depolarization decreases mitochondrial Ca2+ take-up and so anticipates excitotoxic [Ca2+]mit over-burden (3). Mdivi-1 in part depletes ER Ca2+ store and improves peIF2α phosphorylation (ISR; 4), occasions that give cytoprotection against a assortment of stretch conditions.
Mitochondria are exceedingly energetic organelles that always experience parting and combination occasions to adjust to changes within the cellular environment. Distorted mitochondrial parting has been related with a few sorts of cardiovascular brokenness; hindrance of pathologically distorted mitochondrial parting has been appeared to be cardioprotective. Obsessive parting is intervened by the intemperate actuation of GTPase dynamin-related protein 1 (Drp1), making it an appealing restorative target in various cardiovascular infections. Mitochondrial division inhibitor (mdivi-1) is broadly utilized little atom detailed to repress Drp1-dependent parting, prolong mitochondria, and moderate harm. In general, the novel pleiotropic impact of mdivi-1 in cardiomyocytes included proteolytically cleaved L-OPA1, changed expression of OXPHOS complex proteins, and expanded superoxide generation, which together brought about in surrenders in mitochondrial breath and restraint of macro-autophagy.
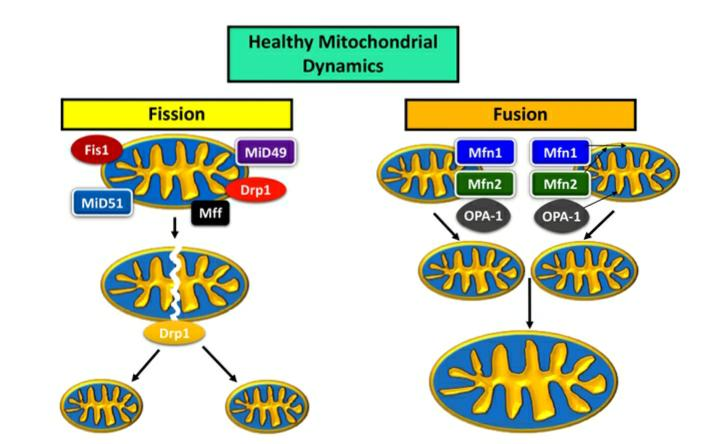
Figure 3: Mitochondrial dynamics in a healthy neuron. Mitochondrial combination and parting have a energetic relationship, with an recede and stream between the expression of combination and parting proteins keeping up a solid mitochondrial organize inside the cell, decided by estimate, dispersion, and biogenesis [4] (Figure 1). The most proteins are Drp1, Mitochondrial parting calculate (MFF), Fission-1 (Fis1), and the homologues Mid49 and Mid51 for the parting pathway; mitofusins 1 (Mfn1) and 2 (Mfn2), and optic decay 1 (OPA1) are proteins that usher mitochondria along the combination pathway.
Treatment with Mdivi-1 at high concentrations (50 μM) has been associated with mitochondrial depolarization, oxidative stress, and apoptosis in oligodendrocytes under excitotoxic conditions, indicating potential adverse effects on mitochondrial function. Treatment with Mdivi-1 at high concentrations (50 μM) has been associated with mitochondrial depolarization, oxidative stress, and apoptosis in oligodendrocytes under excitotoxic conditions, indicating potential adverse effects on mitochondrial function. Mdivi-1 has been shown to induce a moderate inhibition of mitochondrial respiration and a drop in mitochondrial membrane potential, which could have implications for cellular energy production and overall mitochondrial function. Studies suggest that Mdivi-1 may not directly bind to human Drp1 but could interact with human isoform 3 Drp1 at certain concentrations, indicating a complex relationship that may lead to unpredictable outcomes and potential side effects.
These studies underscore the diverse potential benefits of Mdivi-1 in different contexts, from stress tolerance in plants to mitigating inflammatory diseases and neural damage. The compound's ability to modulate mitochondrial function opens avenues for further research into its therapeutic applications.














Comments