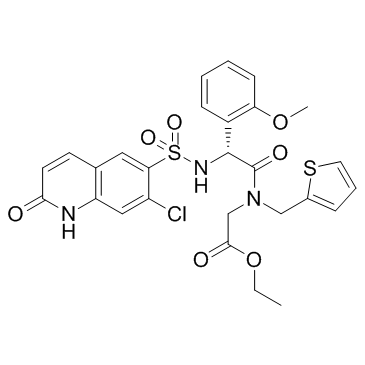
OSMI 4
OSMI 4 is a small, synthetic and novel molecule. It has been discovered in a high-throughput screening (HTS) based on fluorescence luminescence. OSMI 4 is also known as OSMI-4b. The two-dimensional view of the Molecular structure of the OSMI 4 compound is diagrammatically depicted in Figure 1.
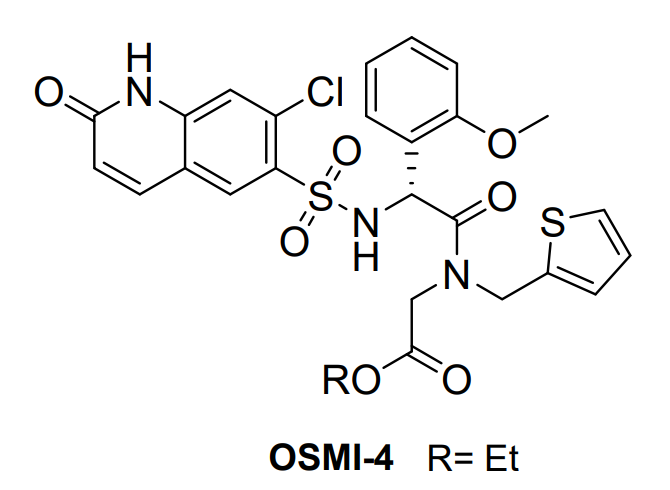
Figure 1 The two-dimensional view of the Molecular structure of the OSMI 4 compound. In this chemical structure of OSMI 4, R represents Ethyl functional group that is shortened as Et
OSMI 4 specifically interacts and effectively inhibits O-GlcNAc transferase (OGT), currently used in studying role of OGT in cell.
The O-GlcNAc transferase (OGT) is composed of different functional domains including TPR13, the transitional helix (H3), the N-Cat domain, the Int-D domain, and the C-Cat domain. Its schematic representation, ribbon representation, three-dimensional structural model in close and open configuration are given in Figure 2, Figure 3, Figure 4, Figure 5, respectively with a uniform color coding in Figure 2, Figure 4, Figure 5, Figure 6 and Figure 7.
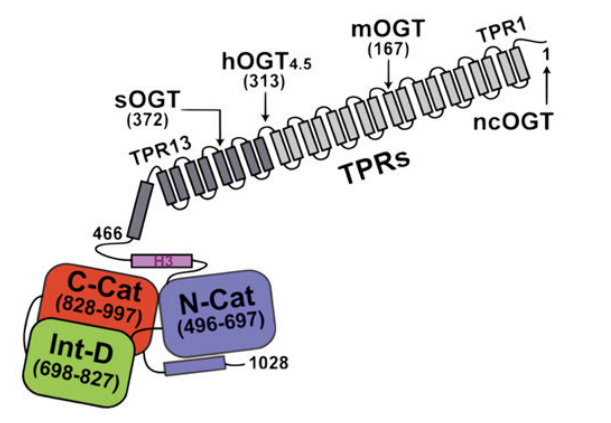
Figure 2 A general Schematic representation of the O-GlcNAc transferase (OGT) molecule indicating the different functional domains present in the O-GlcNAc transferase (OGT)molecule. The lengths of all other isoforms of the O-GlcNAc transferase (OGT) molecule, i.e., sOGT, mOGT, and ncOGT, are also indicated.
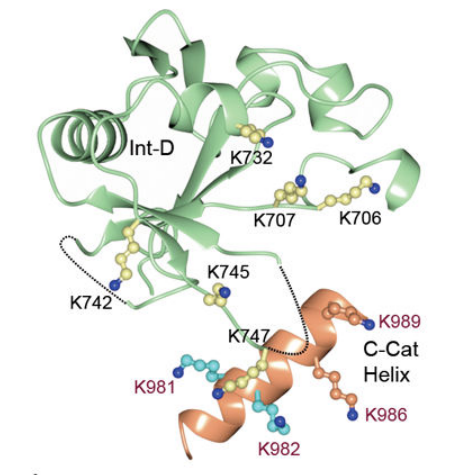
Figure 3 The ribbon representation of three-dimensional crystal structure of O-GlcNAc transferase (OGT) monomer (one single molecule)
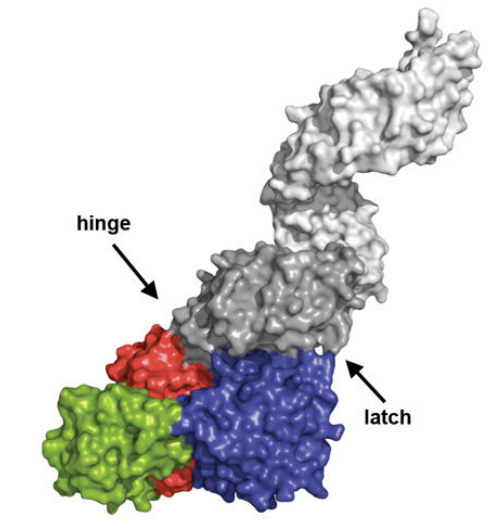
Figure 4 The model of three-dimensional crystal structure of O-GlcNAc transferase (OGT) monomer (one single molecule)
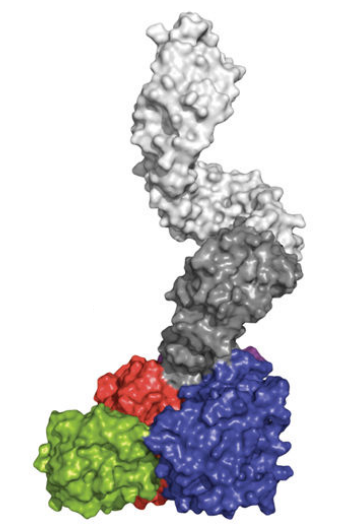
Figure 5 The model of three-dimensional crystal structure of O-GlcNAc transferase (OGT) monomer (one single molecule)
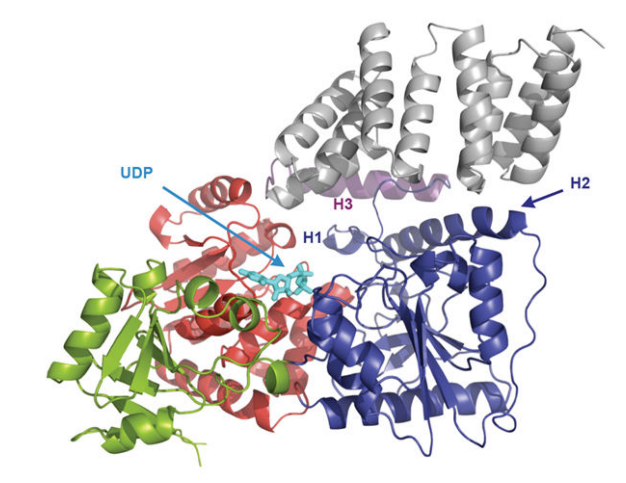
Figure 6 The ribbon representation of the O-GlcNAc transferase (OGT)-Uridine diphosphate complex where different functional domains of O-GlcNAc transferase (OGT) are indicated in different colors and Uridine diphosphate is indicated in cyan; the three-dimensional crystal structure of the O-GlcNAc transferase (OGT) is clear. This molecular complex is transiently formed as N-acetyl glucosamine (GlcNAc) part of the Uridine diphosphate-N-acetyl glucosamine (UDP-GlcNAc) molecule is effectively added to the target protein. TPR units are indicated in gray, the transitional helix (H3) in purple, the N-Cat domain in blue, the Int-D domain in green, and the C-Cat domain in red. Lys842 has a pivotal role in stabilizing the Uridine diphosphate (UDP) in this O-GlcNAc transferase (OGT)-Uridine diphosphate complex
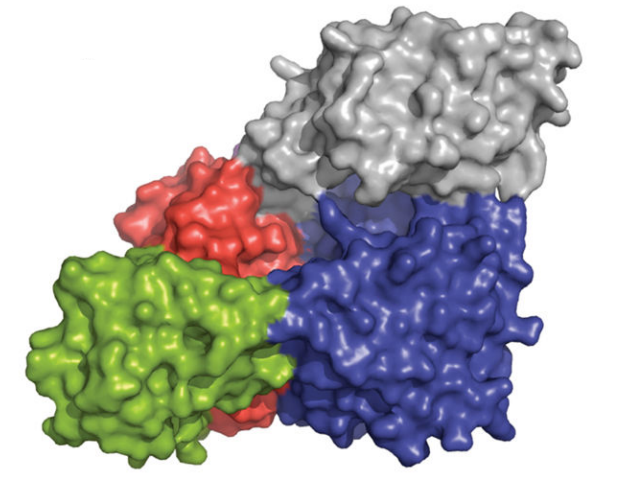
Figure 7 The surface representation of the O-GlcNAc transferase (OGT)-Uridine diphosphate complex where different functional domains of O-GlcNAc transferase (OGT) are indicated in different colors; TPR units are indicated in gray, the N-Cat domain in blue, the Int-D domain in green, and the C-Cat domain in red.
The O-GlcNAc transferase (OGT); as the name indicates, it is an enzyme, i.e., a tertiary level globular structure composed of a linear sequence of covalently bounded amino acids via peptide linkages that is naturally present in the cell as a result of gene expression and acts as a catalyst for a particular type of biochemical reaction. OGT catalyzes the O-Linked β-N-acetylglucosamine glycosylation that is shortened as O-GlcNAcylation, a specialized biochemical reaction that happens after the protein is expressed and folded. In genetics, this type of biochemical reactions is called as post-translational modifications.
In O-GlcNAcylation, N-acetylglucosamine is taken from UDP-GlcNAc and biochemically added specifically at the serine and threonine residual amino acids of the target protein molecules via the formation of O-glycosidic bond. The underlying chemistry of this process which is the core mechanism by which interacts and modifies a huge number of proteins, is depicted in Figure 8. Lys842 stabilizes the Uridine diphosphate (UDP) and thus is critical for the overall catalytic activity of O-GlcNAc transferase (OGT). Naturally, OGA works in opposition to OGT to maintain a homeostatic balance phosphorylation and O-GlcNAcylation. For O-GlcNAcylation, the target proteins identified so far include all the three major types of intracellular protein, i.e., cytosolic proteins, nuclear proteins and mitochondrial protein. Considering their roles in the cell, these target proteins are important signalling enzymes like kinases and phosphatases, transcription factor like tumor suppressor and proteins that support the role of histones in compacting the DNA. After O-GlcNAcylation, one of the most commonly observed point is that those O-GlcNAcylated serine and threonine residuals could not be phosphorylated, i.e., the addition of phosphate group to the target proteins. On the cell level, all the major phenomena including metabolism (catabolism and anabolism), signalling (intracellular and extracellular), transcription (controller of the gene expression and determiner of the gene expression profile of the whole cell), cell cycle (chain of event leading to proliferation and growth), proteostasis (that maintains a healthy amount of fully functional proteins within the cell while degrading all the faulty ones) and lipid droplet formation are performed in a fully regulated manner by a regulated interplay of OGT and OGA. This interplay affecting the overall cell physiology is depicted in Figure 9.
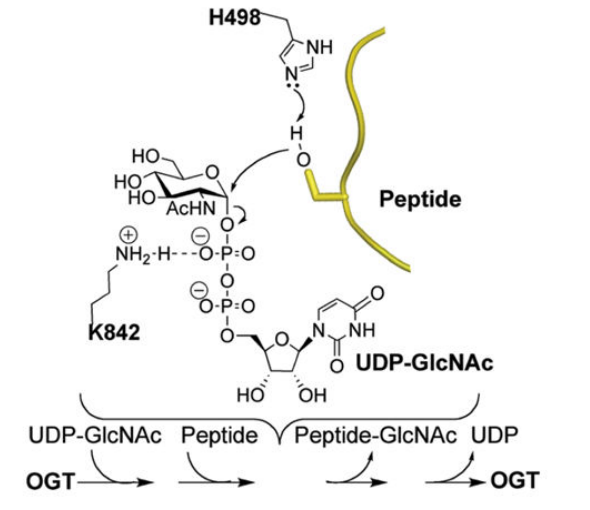
Figure 8 The underlying chemistry/ mechanism of the the O-GlcNAc transferase (OGT) catalysed O-GlcNAcylation is illustrated. In this post translational modification, Uridine diphosphate-N-acetyl glucosamine (UDP-GlcNAc) and the target peptide/ protein shown in yellow are the biochemical reactants while the O-GlcNAc transferase (OGT) acts as biocatalyst; on the other hand, the target protein/ peptide having chemically bounded the N-acetylglucosamine, and Uridine diphosphate (UDP) are the biochemical products. For this reaction, the target protein/ peptide must have at least one reactive hydroxyl group of serine residual.
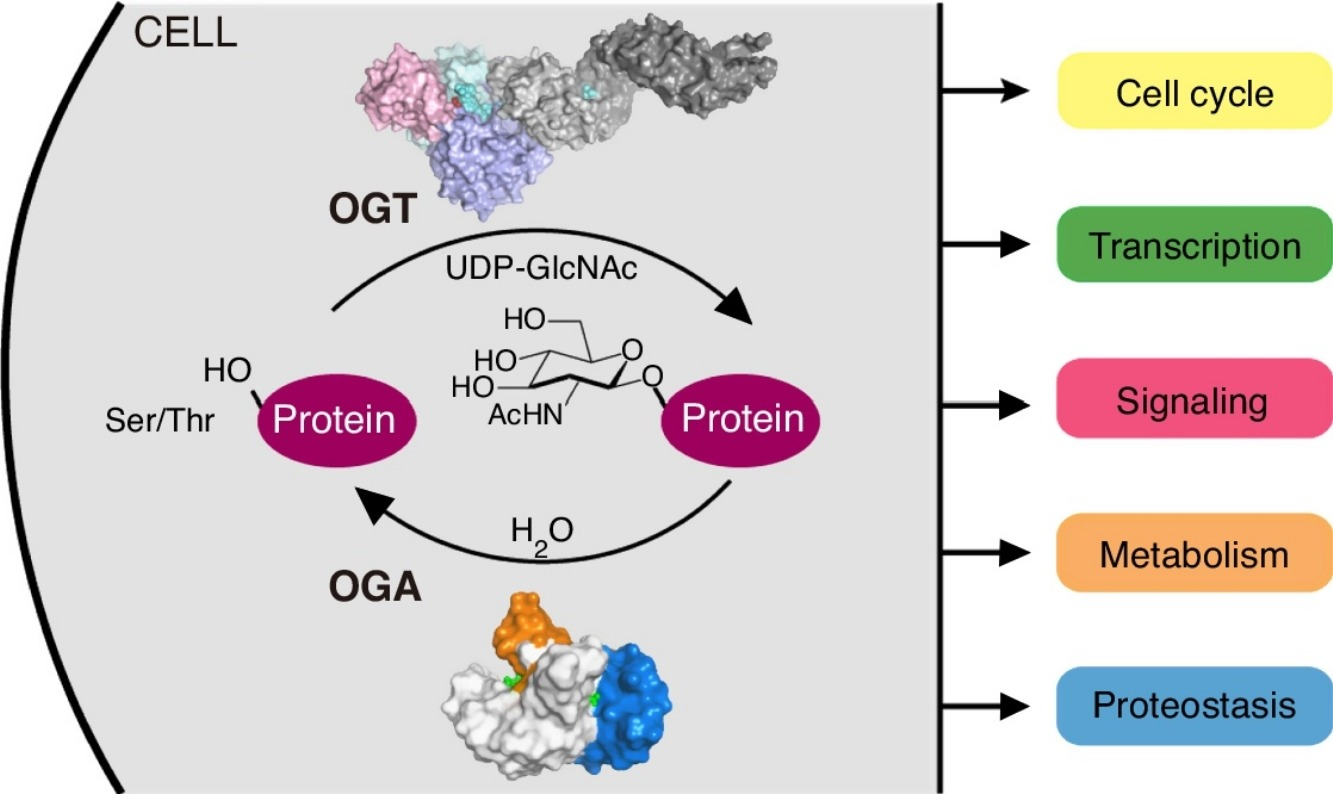
Figure 9 The biological roles of the O-GlcNAc transferase (OGT) within the normal cells
From the clinical perspective, O-GlcNAcylation could also be thought as an index of total cellular metabolism or trophic status or nutrient sensor; as the actual substrate for the O-GlcNAcylation is Uridine diphosphate-N-acetyl glucosamine (UDP-GlcNAc) which comes directly from the hexosamine biosynthetic pathway (HBP), a pathway that collects outputs from the metabolism of all the small molecules namely glucose, amino acid, fatty acids and nucleotides. This idea is illustrated in Figure 10. More glycolysis would yield more Uridine diphosphate-N-acetyl glucosamine (UDP-GlcNAc) and ultimately O-GlcNAcylation would be greater and this again indicates the presence of cancer/ tumors.
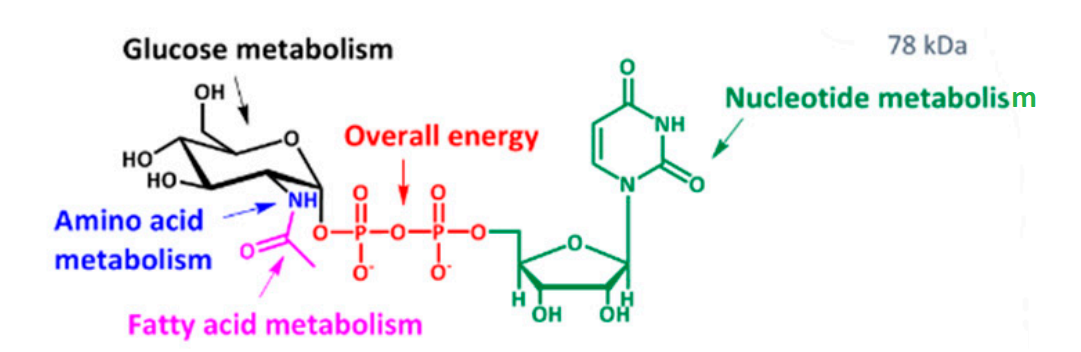
Figure 10 A complete picture of the molecular structure of Uridine diphosphate-N-acetyl glucosamine (UDP-GlcNAc) compound highlighting different functional groups as its structural subunits; it contains glucose moiety indicated in black that comes from glucose metabolism, amino group indicated in blue that comes from amino acid metabolism, acetyl group indicated in magenta that comes from fatty acid metabolism, diphosphates indicated in red that comes from Adenosine Tri Phosphate (ATP) metabolism and Uridine indicated in green that comes from nucleotide metabolism. Thus, it could be easily understood that why Uridine diphosphate-N-acetyl glucosamine (UDP-GlcNAc) compound is considered as an index of total cellular metabolism or trophic status or nutrient sensor
In the pathological conditions, O-GlcNAc transferase (OGT) exhibits a dysregulated expression, i.e., upregulated expression or downregulated expression. The abnormally upregulate O-GlcNAc transferase (OGT) leads to the formation of a tumors marked by uncontrolled proliferation, invasion and metastasis. For example, breast cancer cell, hepatocellular carcinoma. Moreover, several chronic diseases also occur marked by insulin resistance in the adipose and skeletal muscle. For example, type II diabetes (TIID). On the other hand, the abnormally downregulated O-GlcNAc transferase (OGT) is a feature of Alzheimer's disease.













Comments
You should provide the reference for the initial discovery of this and all other inhibitors you discuss/sell. My postdocs, students, and collaborators who worked on the discovery of this compound would appreciate it and it is the right thing to do.