Thapsigargin
Thapsigargin (shortened as Tg) is a small yet structurally complex molecule, that inhibits the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) at the concentration of nano Molar. Tg at this concentration is particularly safe for the other transmembrane pumps It is the extensively studied SERCA inhibitor. The structure of carbon backbone in the Tg is very complex as there lies a fusion of three rings in the heart of its molecular structure. These three rings are; γ-lactone, cycloheptene and cyclopentene. The molecular structure is diagrammatically depicted in the Figure 1. Different ways to synthesize Tg in the laboratory have been proposed in the scientific literature.
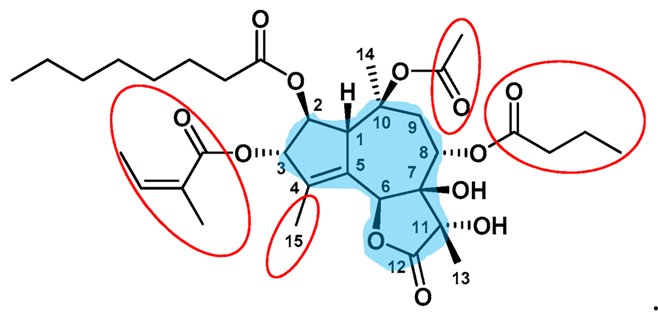
Figure 1 The Molecular Structure of Thapsigargin (shortened as Tg). The three rings, i.e., (1) γ-lactone, (2) cycloheptene and (3) cyclopentene, have been highlighted in the blue area.
SERCA is actually a Ca2+ pump, that is driven by the energy yielded through the hydrolysis of ATP molecule. From the hydrolysis of one molecule of ATP, two calcium ions are transported. During this transport, conformational changes occur to SERCA. At start, SERCA in E1 state, two calcium ions bind to it. Then, SERCA changes its conformation state to E2 and those two calcium ions are transported. Its primary role in any cell to transport the calcium ions; to the sarcoplasmic reticulum (in muscle cells) and the endoplasmic reticulum (in the cells except the muscle cells). This leads to a higher Ca2+ concentration in the sarco/endoplasmic reticulum and a lower Ca2+ concentration in the cytoplasm of the cell. This difference in the Ca2+ concentration within a cell, is critical to the normal physiological functions of that cell, like proliferation and apoptosis, transcription of gene and folding of proteins, etc.
The underlying mechanism of Tg mediated SERCA inhibition is quite simple. When the SERCA is in E2 state, Tg irreversibly binds to it, and forms an inhibitory complex, which deprives SERCA of its ability to bind with two calcium ions and catalytic activity.
Inhibited SERCA leads to the reversal of the Ca2+ concentrations in the sarcoplasmic reticulum (in muscle cells) and the endoplasmic reticulum (in the cells except the muscle cells), which causes the condition known as sarco/endoplasmic reticulum stress. This is also characterized by the incomplete or misfolding of the proteins being synthesized by the ribosomes of the sarco/endoplasmic reticulum. This stress initiates unfolded protein response (UPR), which is essentially a signalling pathway, that ultimately results in either restoring the folding of proteins or killing those cells through apoptosis if the stress remains unresolved. The apoptosis may occur either intrinsically or extrinsically. The unfolded protein response (UPR) is really the decision maker, whether those stressed-out cells are going to live or die. Apart from this, metabolism of Ca2+ negatively shifts which leads to various kinds of pathologies, for examples, neoplasms (commonly known as cancers), diabetes, various muscular diseases, and autoimmune disorders. This Tg mediated sarco/endoplasmic reticulum stress and the subsequently initiated (apoptotic) pathway, i.e., unfolded protein response (UPR), is the basis for using Thapsigargin (Tg) as a therapeutic agent against various types of the neoplasms. Although the exact mechanism of the initiation of the unfolded protein response (UPR) is still unknown. In this therapy using Tg against a wide spectrum of neoplasms, the role of death receptor 5 (DR5) and caspase-8 has found to be very important.
The Tg based therapy has an advantage over other available therapies, that it targets cancer cell equally irrespective of the rate of proliferation. Unlike other therapies, in which the agent targets only those cells which are proliferating at a high rate. Although Tg seems a good candidate for cancer therapy but due to the presence of SERCA in all the cell types. Tg attacks all the body cells, thus has a general toxic effect on all the body cells. To nullify this cytotoxic effect of Tg, pro drug strategy has successfully been developed. The cancer cells express certain proteins, especially enzymes belonging to the proteases class, on their surfaces. In this pro drug strategy, the originally therapeutic agent Tg is conjugated with the substrates of these proteins, which essentially implies that only cancer cells would be able to digest these substrates and Tg would only affect those cells in the body. In this way, although all the body cells have indirectly been exposed to Tg, but only cancerous cell would be in direct exposure to the Tg. For example, cancerous prostate cells express these two proteins, namely, Prostate-specific antigen (PSA) and Prostate specific membrane antigen (PSMA). So, to specifically target cancerous prostate cells, Tg incorporated in the substrate for either of these proteins can be used. In fact, two drugs have been successfully developed, employing this pro drug strategy. These drugs include G115 against prostate cancer and G202 against prostate cancer, breast cancer and bladder cancer. In both of these drugs, derivatives of Tg have been employed instead of Tg as native Tg does not have any site of attachment. For G115, Leu-12ADT is employed, while for G202, βAsp-12ADT is employed. Out of these, G202 has been successful to enter phase 2 of clinical trials.
The Tg role against leukemia has been shown in the human T cell acute lymphoblastic leukemia (T-ALL), which is marked by the presence of the mutated NOTCH1 gene and NOTCH receptors. And, Tg selectively and effectively functionally inhibits these mutated NOTCH receptors in these T cell acute lymphoblastic leukemia (T-ALL). As discussed earlier, Tg has a general cytotoxic effect to every cell of the body. Therefore, a prodrug, namely, JQ-FT has been introduced for the treatment of leukemia. As leukemia cells express folate receptors (FRs) on their surface, Tg derivative (alcohol derivative, namely 8-debutanoyloxythapsigargin) has been conjugated with folic acid. JQ-FT has shown positive therapeutic effects in in vitro as well as in vivo studies (Jaskulska et al., 2021). The molecular structure of this pro drug is diagrammatically depicted in the Figure 2.
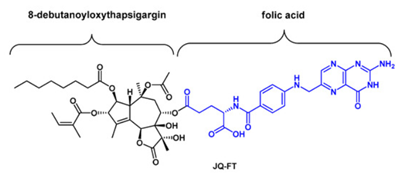
Figure 2 The Pro Drug, namely, JQ-FT using Alcohol derivative of Tg and Folic Acid covalently bonded through an ester bond
Apart from acting as an anti-cancer drug, Tg also kills Zika virus (ZIKV) which causes infection that in return is the cause of various pathologies. Tg eliminates the viral replication via the similar mechanism as it acts an anti-cancer drug. The mechanism includes putting the infected cell to the sarco/endoplasmic reticulum stress by rupturing the Ca2+ concentrations within the cytoplasm (abnormally high concentration). And, the subsequent apoptosis of these stressed cells. Interestingly, in this research study, it has also been found that targeting sarco/endoplasmic reticulum is the most effective way to destroy the Zika virus (ZIKV) as compared to targeting other cellular organelles (Ojha et al., 2023).
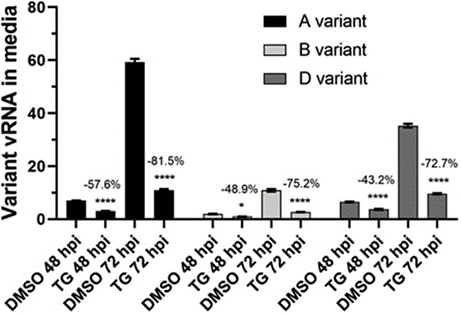
As we all know, Coronavirus Disease (COVID 19) had become very challenging owning to the presence various variants of the corona virus, i.e., alpha, beta and delta. But, fortunately, Tg has been found to effectively demonstrate anti-viral effects against different variant of corona virus. Surprisingly enough, the anti-viral effects have also been seen when different variants are present in different combinations. This is verily evident from the graphical presentation in the Figure 3. The anti-viral effects were observed as Tg reduced the synthesis of RNA, thereby inhibiting the replication of corona virus (Al-Beltagi et al., 2021).
Figure 3 The reduced concentration of mRNA of different variants of Corona Virus; alpha, beta and delta. Tg has the best anti-viral effect as compared to others












Comments