TRASTUZUMAB
Another name for trastuzumab is Herceptin. Trastuzumab is an antibody, i.e., a protein molecule that essentially means being composed of amino acids and has a major key role in providing the body with immunity, which is artificially produced in the laboratory by the monoclone cell/ monoclones; thus, it is also regarded as an immunoglobulin or it can also be more appropriately termed as monoclonal antibody which is shortened as mAb. Trastuzumab is a humanized antibody, i.e., the protein sequence was originally taken from some non-human biological species, then its sequence is modified such that it becomes extremely similar to the antibodies that are naturally produced in the human body in terms of structure. In the humanized antibodies, the protein sequence that belongs to the non-human biological species is the functional region while the introduced human antibody like region keeps the overall antibody biochemically stable while protecting it from the natural immune response of the human body. Being an antibody, Trastuzumab follows the generic structure of an antibody which is schematically presented in Figure 1.
Figure 1 The generalized structure of an antibody. Here, it is presented as schematically equivalent of Trastuzumab
Trastuzumab interacts and binds to Human epidermal growth factor receptor 2 (HER2) that is also called as ErbB receptor tyrosine kinase 2 (ErbB2), which belongs to the protein family of Human epidermal growth factor receptors (HER).
The general structure that is followed by all the protein members of the Human epidermal growth factor receptors (HER) family includes three major domains, first an extracellular domain lying outside of the cell surface consisting of four subdomains and mainly involved in the dimerization of the Human epidermal growth factor receptors (HER), second a transmembrane domain embedded in the hydrophobic core of the plasma membrane lipid bilayer connecting the extracellular domain and the cytoplasmic domain and third a cytoplasmic domain lying on the inside of the cell surface consisting of tyrosine kinase and other subdomains which are primarily involved in the regulatory processes.
Human epidermal growth factor receptors (HER) which further belongs to the protein class of Receptor tyrosine kinases (RTKs), i.e., as the name indicates these are the receptor protein which takes phosphate group from some energy molecule, most popular example would be ATP and then adds that phosphate group to the target molecule specifically at the positions of tyrosine amino acid in active state. No natural ligand has been discovered for Human epidermal growth factor receptor 2 (HER2); Human epidermal growth factor receptor 2 (HER2) mediates the signalling by just being involved in the dimerization. Interestingly, all the members of the protein family, i.e., Human epidermal growth factor receptors (HER), interact and has the potential to make dimers with one another. These dimers could be homo or hetero in nature; through their formation, all the vital cellular processes are regulated namely cellular growth, cellular differentiation and even overall cellular survival is regulated via the initiation of several intracellular signalling pathways in which the phosphorylation of the tyrosine residual amino acids is the most common feature. After the phosphorylation step, several new sites for interaction with the downstream effector molecules appear on the Human epidermal growth factor receptors (HER) protein molecule. These effector molecules commonly include Growth factor Receptor-Bound protein 2 (Grb2) and Src Homology 2 domain containing transforming protein (Shc) as indicated by 1 and 2 in Figure 2; these are the same proteins that are involved in the regulation of mitogen-activated protein kinase (MAPK) signaling pathway. Moreover, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/AKT) signaling pathway also get activated when there is a formation of a heterodimer between one molecule of Human epidermal growth factor receptor 2 (HER2) and one molecule of Human epidermal growth factor receptor 3 (HER3). The latter molecule is phosphorylated at multiple tyrosine residual amino acids thus providing multiple interaction sites for the p85 subunit of phosphatidylinositol 3-kinase (PI3K). This is the direct activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/AKT) signaling pathway. However, there is also an indirect way of activating the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/AKT) signaling pathway mediated by the Human epidermal growth factor receptor 2 (HER2). This activation is Src-mediated and in this way, the activity of the phosphatase and tensin homolog (PTEN) is inhibited as indicated by 3 in Figure 2. The overall summary of all the signaling taking place downstream of the Human epidermal growth factor receptors (HER) protein molecule is schematically presented in Figure 2.
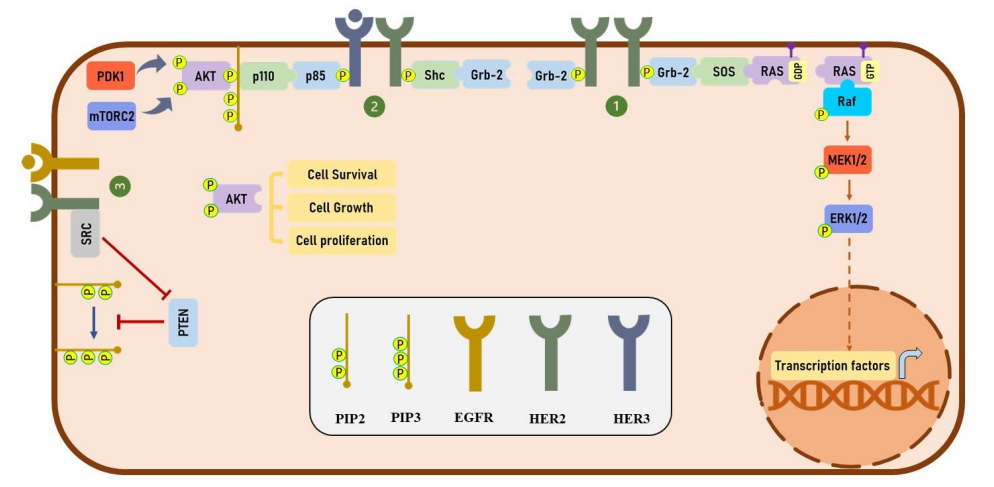
Figure 2 The signalling pathways which are present downstream of the Human epidermal growth factor receptors (HER) protein molecule. In small box, the legends used for the different proteins are given, i.e., PIP1 stands for , PIP3 stands for , EGFR stands for , HER2 stands for and HER3 stands for . Out of them, only EGFR, HER2 and HER3, as belonging to the Human epidermal growth factor receptors (HER), are capable of forming homodimers as well as heterodimers. Phosphorylation followed by the dimer formation, ultimately leads to the activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/AKT) signaling pathway; and thereby all the vital cellular processes are regulated that leads to the cell survival, growth and differentiation.
On the other hand, under the pathological condition like in cancer Human epidermal growth factor receptor 2 (HER2) is abnormally overexpressed; and inhibiting the Human epidermal growth factor receptor 2 (HER2) dimerization by the introduction of Trastuzumab is the strategy to overcome the cancers/ neoplasms involving the overexpressed Human epidermal growth factor receptor 2 (HER2). Apart from breast cancers, gastric cancer, non-small cell lung cancer (NSCLC) and colorectal cancer are such neoplasms.
The U. S. Food and Drug Administration (FDA) has only approved the Trastuzumab alone as a pharmaceutical drug for the treatment of breast cancer in the metastasis state in the clinical settings. Although, several derivative formulations also exist that used Trastuzumab as a main drug and other components as well. One such example is T-DXd, which combined Trastuzumab and Deruxtecan, with satisfactory clinical response and safety profile. Although, this formulation is not approved by the U. S. Food and Drug Administration (FDA) yet.
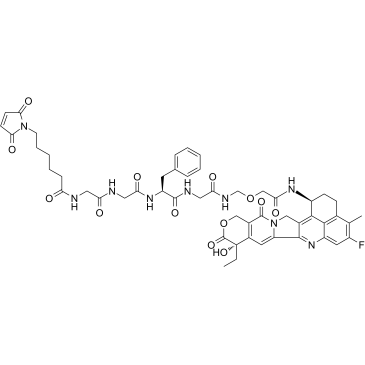
The structural schematic for the T-DXd is presented in Figure 3. It specifically interacts and binds to the Human epidermal growth factor receptor 2 (HER2). Upon binding, the Human epidermal growth factor receptor 2 (HER2) along with the Trastuzumab-Deruxtecan molecule is internalized and being consequently attacked by the enzymes of the lysosome within the cytosol that results in the enzymatic degradation of this receptor-ligand complex. This step can be visualized by the cleaved subunits of the receptor-ligand complex. This cleavage is actually clinically significant as it releases Deruxtecan molecule which was previously bounded to Trastuzumab and thus was inactive. But after cleavage, it is now active. Moreover, it can cross the nuclear membrane and cause the DNA damage thus results in apoptosis in the tumor cells, shown in Figure 4.
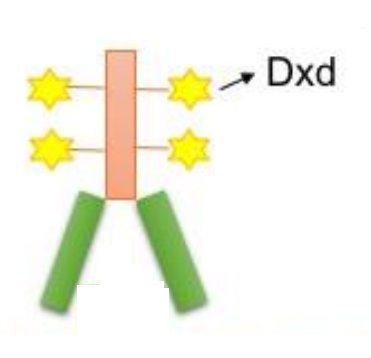
Figure 3 The Molecular Schematic of the Trastuzumab-Deruxtecan or T-DXd. The Deruxtecan or Dxd is indicated by stars attached to the Trastuzumab molecule. It specifically and effectively inhibits the topoisomerase and thus enhance the anti-cancer activity of Trastuzumab
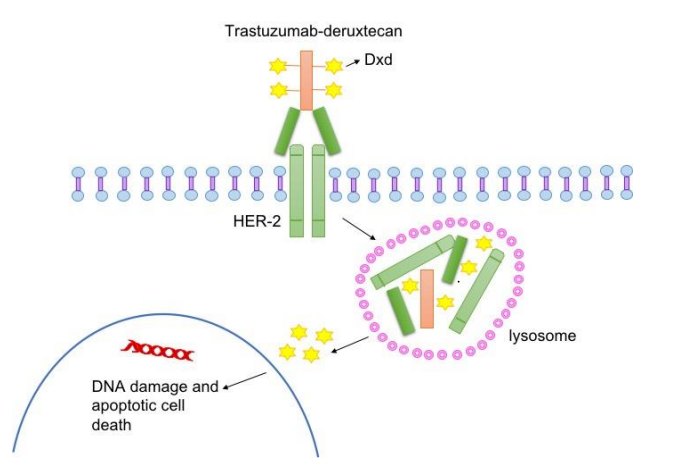
Figure 4 The underlying mechanism of action of Trastuzumab-Deruxtecan or T-DXd














Comments