ML385 |
| Katalog-Nr.GC19254 |
ML385 ist ein spezifischer Inhibitor des nuklearen Faktors Erythroid 2-verwandter Faktor 2 (NRF2).
Products are for research use only. Not for human use. We do not sell to patients.
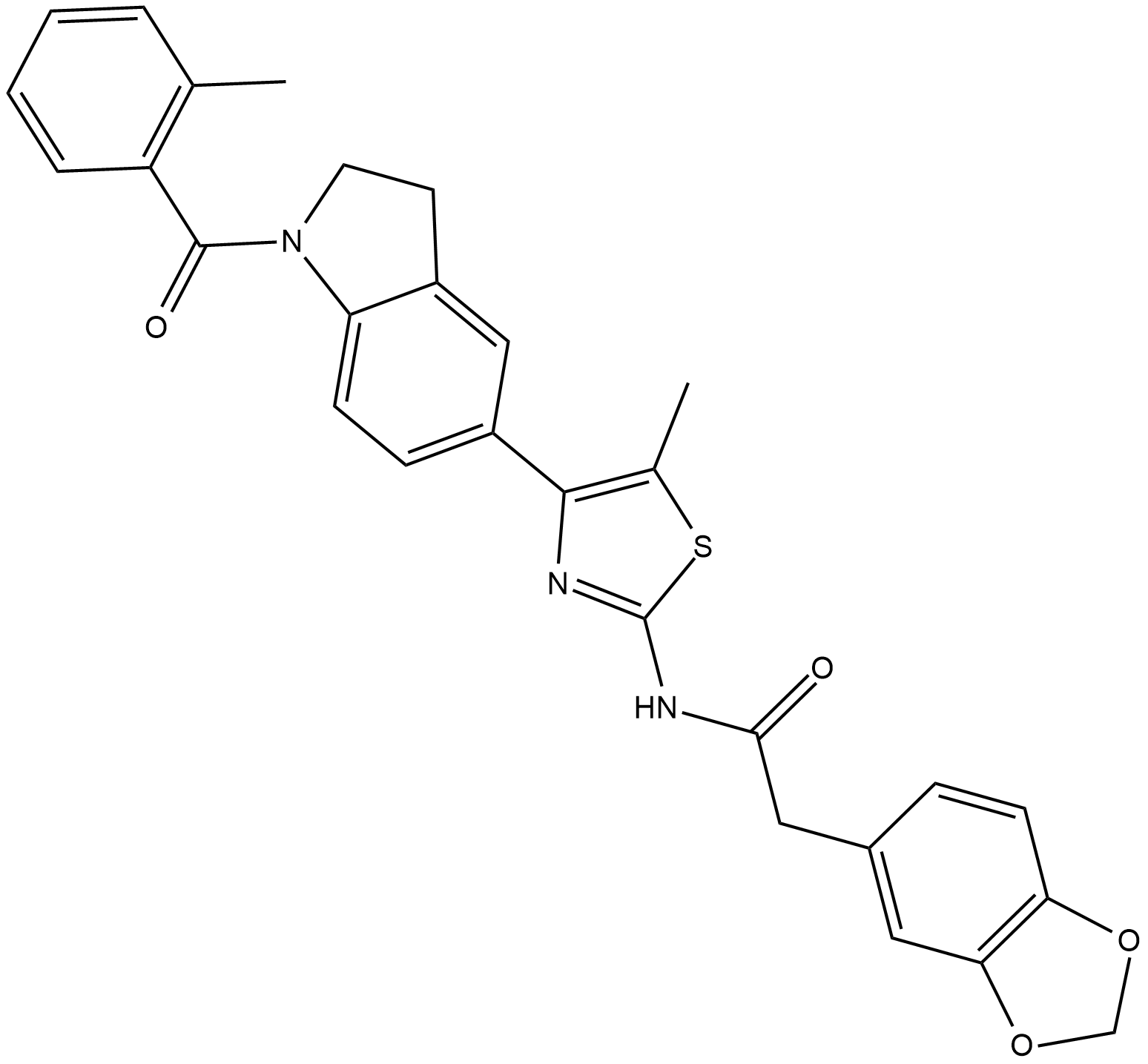
Cas No.: 846557-71-9
Sample solution is provided at 25 µL, 10mM.
ML385 ist ein spezifischer Inhibitor des nuklearen Faktors Erythroid 2-verwandter Faktor 2 (NRF2). ML385 bindet an NRF2 und hemmt als Sondensubstanz die Expression seiner nachgeschalteten Zielgene. Genauer gesagt bindet ML385 an Neh1, die Cap ‘N’ Collar Basic Leucine Zipper (CNC-bZIP)-Domäne von NRF2, und stört die Bindung des V-Maf Avian Musculoaponeurotic Fibrosarcoma Onkogenhomolog G (MAFG)-NRF2-Proteinkomplexes an regulatorische DNA-Bindungssequenzen. ML385 zeigt Spezifität und Selektivität für NSCLC-Zellen mit KEAP1-Mutation, die zu einem Funktionsgewinn von NRF2 führt.[1][2]
Eine In-vitro-Studie zeigte, dass ML385 NRF2 potenziell durch direkte Interaktion hemmt. Das NRF2-Signal nahm zeitabhängig ab, wobei der maximale Rückgang nach 72 Stunden beobachtet wurde. Es wurde auch eine Reduktion der NRF2-mRNA-Spiegel festgestellt. Darüber hinaus verursachte ML385 eine globale Hemmung der NRF2-Signale in Lungenkrebszellen mit KEAP1-Mutationen und anderen Zielgenen. Die Behandlung mit ML385 verringerte auch signifikant die NQO1-Enzymaktivität und reduzierte die GSH-Spiegel sowie die zelluläre Antioxidationskapazität.[2]
In vivo Studien mit ML385 zeigten, dass es NRF2 hemmt und vielversprechende anti-tumorale Aktivitäten aufweist. ML385 in Kombination mit Carboplatin zeigte eine signifikante Reduktion des Tumorwachstums im Vergleich zum Vehikel. Obwohl die Behandlung mit einem einzelnen Wirkstoff (entweder ML385 oder Carboplatin) zu einer Reduktion des Tumorwachstums führte, war das Ausmaß dieser Effekte zwischen den Zelllinien unterschiedlich und erreichte keine statistische Signifikanz. Darüber hinaus zeigten Tumorproben, die mit ML385 behandelt wurden, eine signifikante Reduktion des NRF2-Proteingehalts und seiner nachgeschalteten Zielgene. Außerdem führte die Zugabe von ML385 zu einer dosisabhängigen Verringerung der Anisotropie mit einem IC50 von 1,9 μM, was darauf hindeutet, dass der NRF2-MAFG-Proteinkomplex von fluoreszenzmarkierter ARE-DNA dissoziiert wurde.[2]
References:
[1]. Xian P, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019 Aug 14;9(20):5956-5975.
[2]. Singh A, et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem Biol. 2016 Nov 18;11(11):3214-3225.
Average Rating: 5 (Based on Reviews and 39 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *









