ML385 |
| Catalog No.GC19254 |
ML385 is a specific nuclear factor erythroid 2-related factor 2 (NRF2) inhibitor.
Products are for research use only. Not for human use. We do not sell to patients.
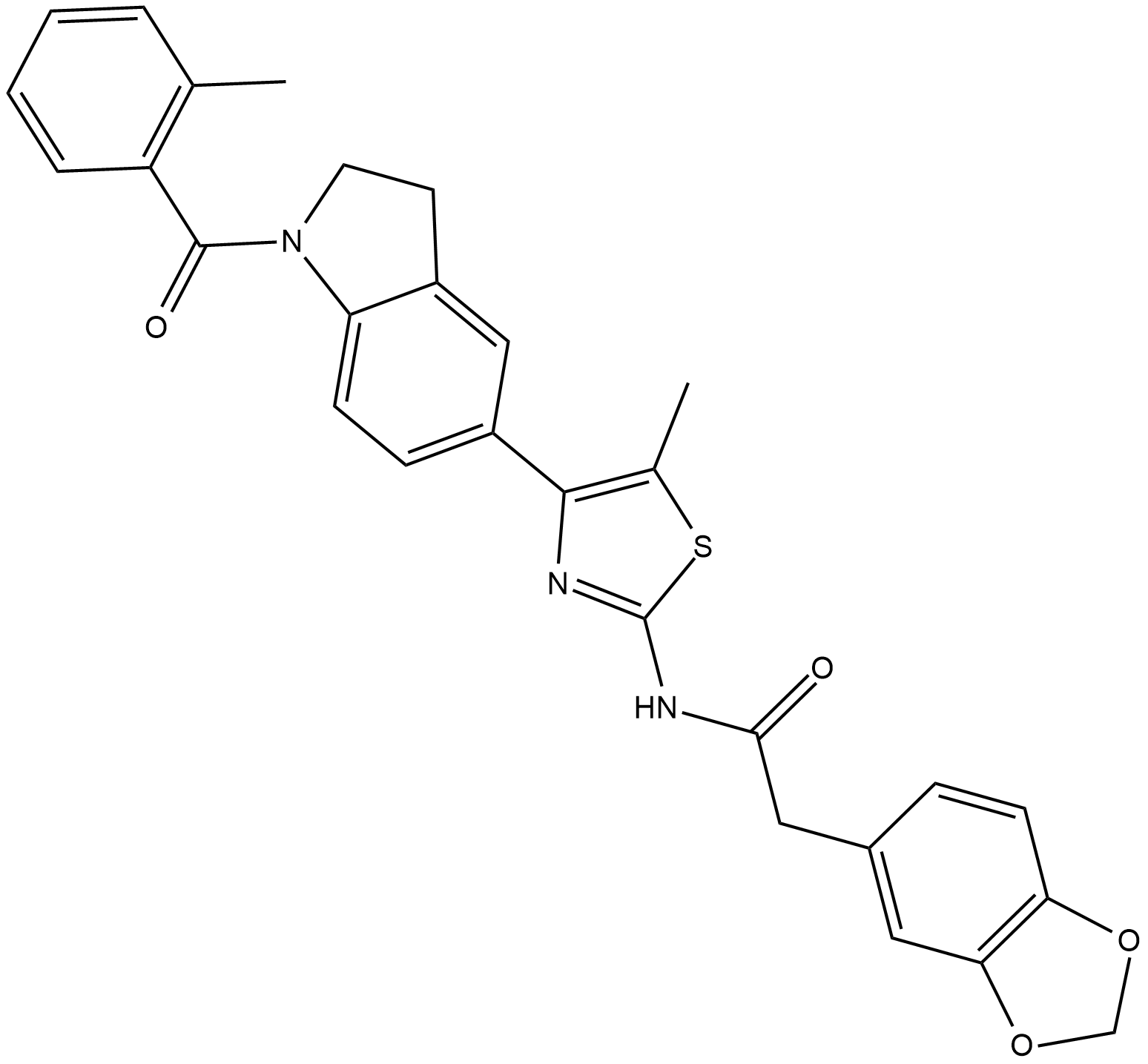
Cas No.: 846557-71-9
Sample solution is provided at 25 µL, 10mM.
ML385 is a specific nuclear factor erythroid 2-related factor 2 (NRF2) inhibitor. ML385 binds to NRF2 and inhibits its downstream target gene expression as a probe molecule. Specifically, ML385 binds to the Neh1, the Cap ‘N’ Collar Basic Leucine Zipper (CNC-bZIP) domain of NRF2, and interferes with the binding of the V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog G (MAFG)-NRF2 protein complex to regulatory DNA binding sequences. ML385 shows specificity and selectivity for NSCLC cells with KEAP1 mutation leading to gain of NRF2 function.[1][2]
In vitro study demonstrated that ML385 potentially inhibits NRF2 through direct interaction. The NRF2 signaling was decreased in a time-dependent manner and the maximum decline was at 72 h. A reduction in NRF2 mRNA levels was also observed. In addition, ML385 caused global inhibition of NRF2 signaling in lung cancer cells with KEAP1 mutations, and other target genes. ML385 treatment also significantly attenuated NQO1 enzyme activity and reduced GSH levels along with cellular antioxidant capacity. [2]
In vivo study of ML385 indicated that it inhibits NRF2 and showed promising anti-tumor activity. ML385 in combination with carboplatin showed a significant reduction in tumor growth compared to vehicle. Although the treatment with a single agent (either ML385 or carboplatin) led to a reduction in tumor growth, the magnitude of these effects was variable between cell lines and did not reach statistical significance. Moreover, tumor samples treated with ML385 showed a significant reduction in NRF2 protein level and its downstream target genes. Besides, the addition of ML385 decreased anisotropy in a dose-dependent manner, with an IC50 of 1.9 μM, suggesting that the NRF2-MAFG protein complex was dissociated from fluorescein-labeled ARE-DNA. [2]
References:
[1]. Xian P, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019 Aug 14;9(20):5956-5975.
[2]. Singh A, et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem Biol. 2016 Nov 18;11(11):3214-3225.
Average Rating: 5 (Based on Reviews and 39 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *








