EPZ-6438 (Synonyms: E-7438,Tazemetostat) |
| Catalog No.GC14062 |
EPZ-6438 is a potent and bio-available inhibitor of EZH2, the catalytic subunit of polycomb repressive complex 2 (PRC2) catalyzing the methylation of lysine 27 of histone H3 (H3K27), that inhibits the activity of human PRC2-containing wild-type EZH2 with a value of inhibition constant Ki of 2.5 nM.
Products are for research use only. Not for human use. We do not sell to patients.
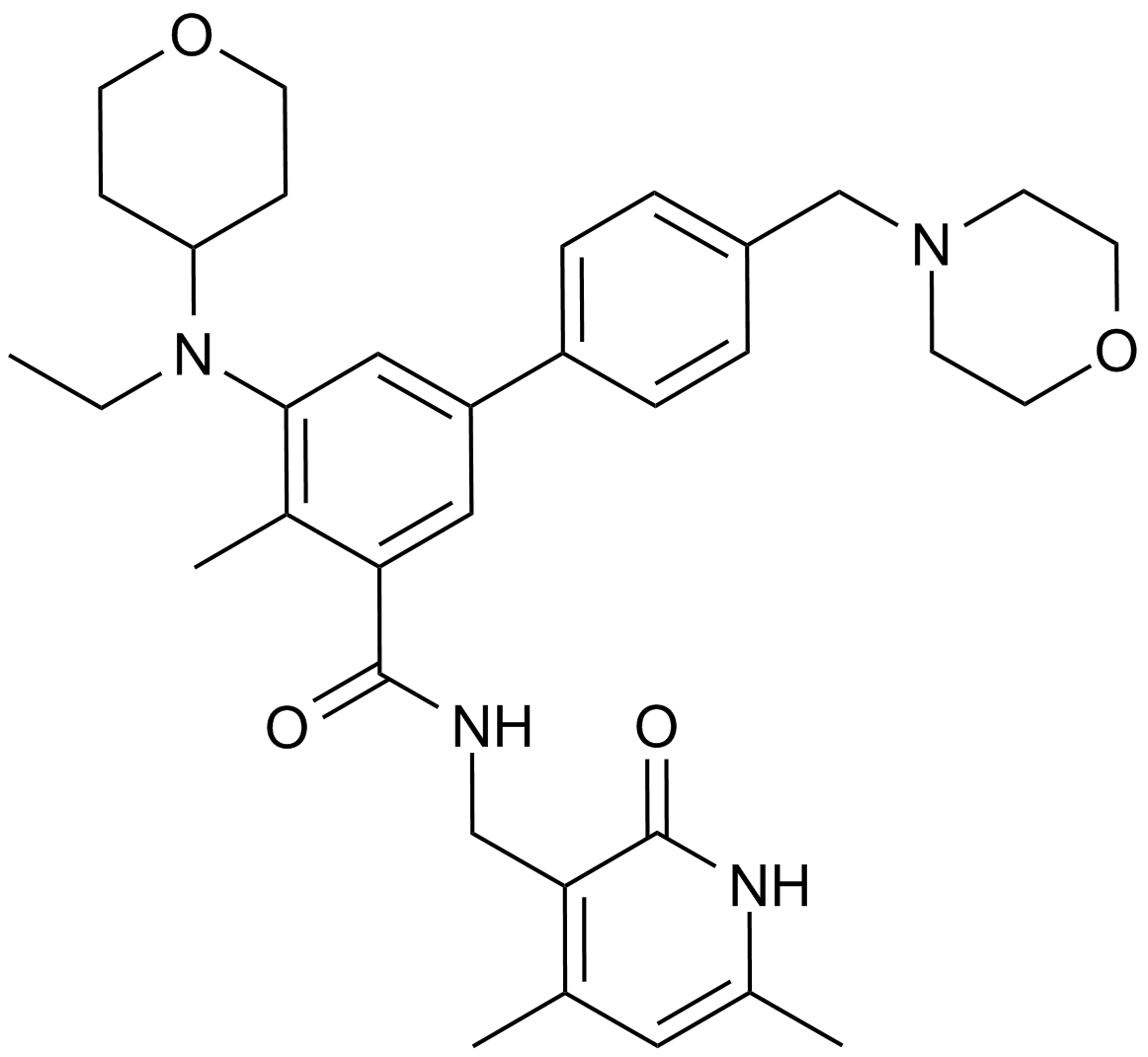
Cas No.: 1403254-99-8
Sample solution is provided at 25 µL, 10mM.
EPZ-6438 is a potent and bio-available inhibitor of EZH2, the catalytic subunit of polycomb repressive complex 2 (PRC2) catalyzing the methylation of lysine 27 of histone H3 (H3K27), that inhibits the activity of human PRC2-containing wild-type EZH2 with a value of inhibition constant Ki of 2.5 nM. EPZ-6438 competitively binds to the S-adenosylmethionine (SAM) binding site of EZH2 and also non-competitively binds to the binding sites of peptide or nucleosome substrate. EPZ-6438 selectively inhibits EZH2 with selectivity 35-fold greater than EZH1. Study results have suggested that EPZ-6438 exhibits dramatic and permanent anti-tumor activity in MRT models through synergistic effects of EPZ-6438-mediated EZH2 inhibition on several cancer pathways.
Reference
[1].Sarah K. Knutson1, Natalie M. Warholic, Tim J. Wigle, Christine R. Klaus, Christina J. Allain, Alejandra Raimondi, Margaret Porter Scott, Richard Chesworth, Mikel P. Moyer, Robert A. Copeland, Victoria M. Richon, Roy M. Pollock, Kevin W. Kuntz, and Heike Keilhack. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. PNAS 2013; 110(19): 7922-7927
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




