Fluspirilene (Synonyms: McN-JR 6218,R 6218,Redeptin) |
| Catalog No.GC11492 |
potent, non-competitive antagonist of agonist-activated L-type calcium channels
Products are for research use only. Not for human use. We do not sell to patients.
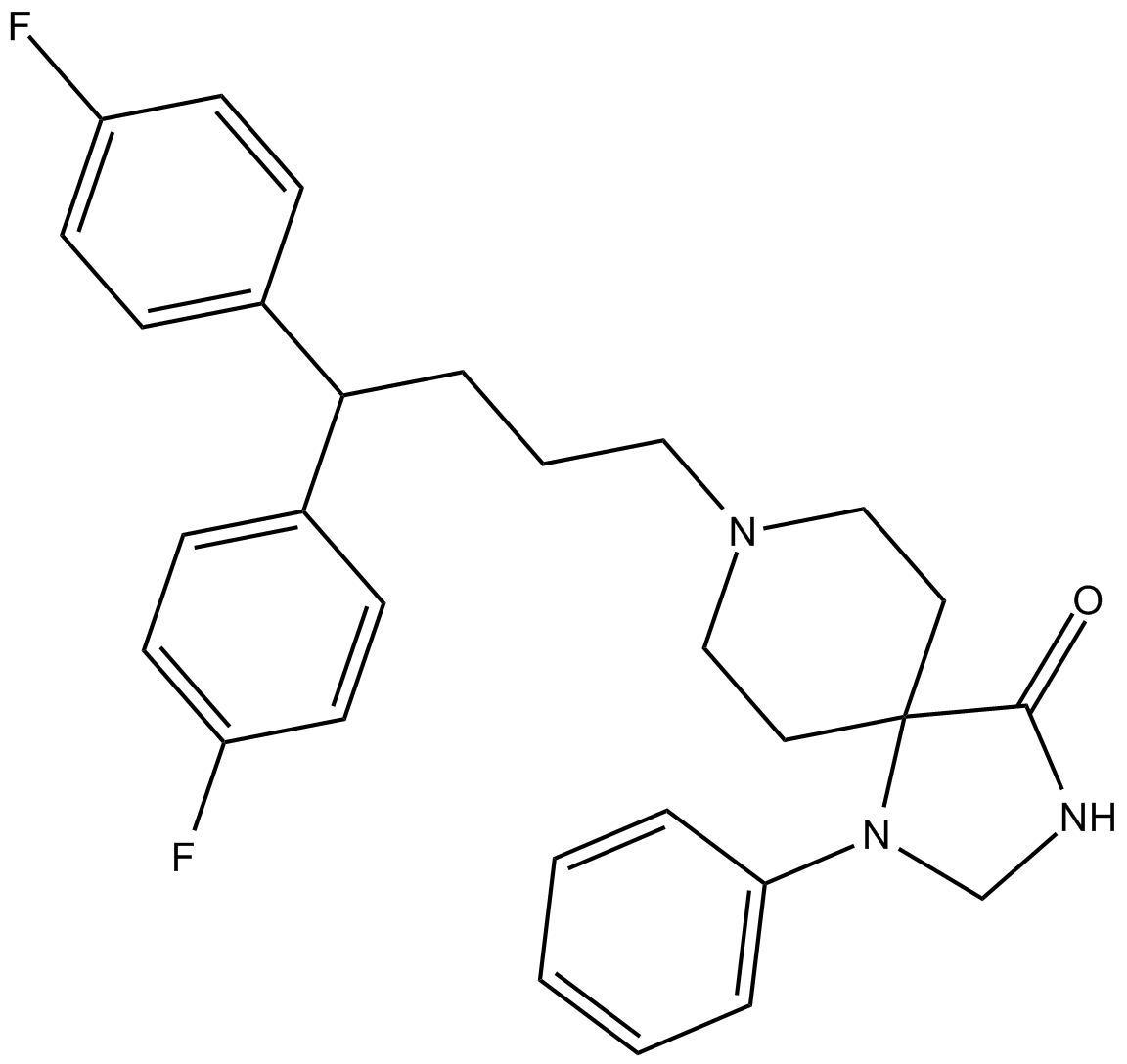
Cas No.: 1841-19-6
Sample solution is provided at 25 µL, 10mM.
IC50: 0.03 μM
Fluspirilene is a potent and non-competitive antagonist of agonist-activated L-type calcium channels.
Voltage-gated calcium channels are critical for coupling membrane depolarization to the influx of calcium. The calcium that flows into excitable cells via voltage-gated calcium channels generates both electrical and chemical signals.
In vitro: Fluspirilene was found to be weakly active as an antagonist of Ca2(+)-induced contractions in K(+)-depolarized taenia. In addition, fluspirilene at 10-1000 nM was a potent non-competitive antagonist of the effects of Bay K 8644 on Ca2(+)-induced contractions and could selectively antagonise the effects of Bay K 8644, without affecting the calcium antagonist effects of nitrendipine [1].
In vivo: In a previous animal study, adult male Wistar rats were intramuscularly injected with a 8 mg/kg dose of fluspirilene. Results showed that the excretion was slow but constant during the first 12 days. The identified metabolites of the urine and faeces showed oxidative N-dealkylation as the major metabolic pathway [2].
Clinical trial: A clinical trial was carried out in which fluspirilene was compared to chlorpromazine in the treatment of schizophrenic patients with acute exacerbation. Similar therapeutic improvement was obtained with both drugs, but men needed a significantly higher mean dose of fluspirilene than women. Fluspirilene induced more parkinsonism than chlorpromazine, but less drowsiness, dizziness, and dry mouth [3].
References:
[1] Kenny, B. A.,Fraser, S.,Kilpatrick, A.T., et al. Selective antagonism of calcium channel activators by fluspirilene. Br. J. Pharmacol. 100(2), 211-216 (1990).
[2] Heykants JP. The excretion and metabolism of the long-acting neuroleptic drug fluspirilene in the rat. Life Sci. 1969 Oct 1;8(19):1029-39.
[3] Chouinard G, Annable L, Steinberg S. A controlled clinical trial of fluspirilene, a long-acting injectable neuroleptic, in schizophrenic patients with acute exacerbation. J Clin Psychopharmacol. 1986 Feb;6(1):21-6.
Average Rating: 5 (Based on Reviews and 1 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















