ABT-239 |
| Catalog No.GC35222 |
ABT-239 is a novel, highly efficacious, non-imidazole?class of H3R antagonist and a transient receptor potential vanilloid type 1 (TRPV1) antagonist.
Products are for research use only. Not for human use. We do not sell to patients.
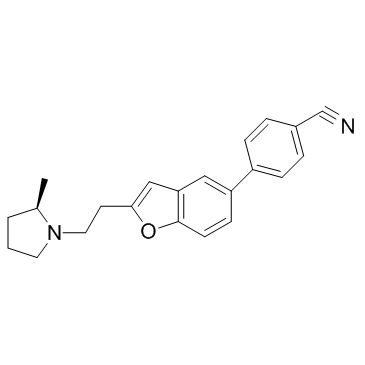
Cas No.: 460746-46-7
Sample solution is provided at 25 µL, 10mM.
ABT-239 is a novel, highly efficacious, non-imidazole class of H3R antagonist and a transient receptor potential vanilloid type 1 (TRPV1) antagonist.
Perfusion of the TMN with ABT-239 (10 μM) increases histamine release from the TMN, NBM, and cortex, but not from the striatum or NAcc. TMN perfusion with ABT-239 activates c-Fos selectively in the NBM and cortex[4].
ABT-239 (3 mg/kg, i.p.) significantly delays onset of seizure, reduces behavioral seizures elicited by KA, and reduces in the incidence of head bobbing and forelimb clonus in mice. ABT-239 (1 mg/kg, i.p.) in conbination with sub-therapeutic dose of SVP (150 mg/kg, i.p.), significantly decreases the number of immobility, head bobbing and forelimb clonus, where as a higher dose combination of ABT-239 (3 mg/kg, i.p.) causes enhanced reduction in all the stages. ABT-239 (3 mg/kg, i.p.) and TDZD-8 (10 mg/kg, i.p.) have more powerful reduction in the number of pyknotic neurons in mice hippocampi. The high dose combination of ABT-239 and TDZD-8 produces the most pronounced increase in Bcl-2 expression as well as decrease in the level of Bax[1]. ABT-239 (3 mg/kg, i.p.) administration transforms a short-term learning event into a long-term remembered experience in WT but not in histamine-depleted mice[2]. Concomitant administration of either ABT-239 (1 and 3 mg/kg, i.p.) and nicotine (0.035 mg/kg, i.p.), or ABT-239 (0.1 mg/kg, i.p.) and nicotine (0.0175 mg/kg, i.p.) further increases nicotine-induced improvement in both memory acquisition and consolidation[3].
[1]. Bhowmik M, et al. Histamine H3 receptor antagonism by ABT-239 attenuates kainic acid induced excitotoxicity in mice. Brain Res. 2014 Sep 18;1581:129-40. [2]. Provensi G, et al. Donepezil, an acetylcholine esterase inhibitor, and ABT-239, a histamine H3 receptor antagonist/inverse agonist, require the integrity of brain histamine system to exert biochemical and procognitive effects in the mouse. Neuropharmacolo [3]. Kruk M, e tal. Effects of the histamine H2 receptor antagonist ABT-239 on cognition and nicotine-induced memory enhancement in mice. Pharmacol Rep. 2012;64(6):1316-25. [4]. Munari L, et al. Selective brain region activation by histamine H2 receptor antagonist/inverse agonist ABT-239 enhances acetylcholine and histamine release and increases c-Fos expression. Neuropharmacology. 2013 Jul;70:131-40.
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















