Vonoprazan |
| Catalog No.GC37920 |
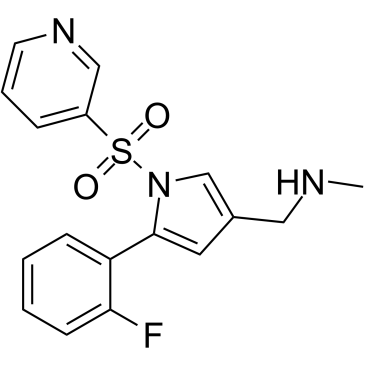
Vonoprazan (TAK-438 free base), a proton pump inhibitor (PPI), is a potent and orally active potassium-competitive acid blocker (P-CAB), with antisecretory activity.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 881681-00-1
Sample solution is provided at 25 µL, 10mM.
Vonoprazan (TAK-438 free base) is an orally active potassium-competitive acid blocker which inhibits H+, K+-ATPase activity with an IC50 of 19 nM.
[1]. Arikawa Y, et al. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem, 2012, 55(9), 4446-4456. [2]. Hori Y, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther, 2010, 335(1), 231-238. [3]. Hori Y, et al. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther, 2011, 337(3), 797-804.
Average Rating: 5 (Based on Reviews and 32 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















