Istaroxime (Synonyms: PST-2744;PST 2744;PST2744) |
| Catalog No.GC11448 |
Products are for research use only. Not for human use. We do not sell to patients.
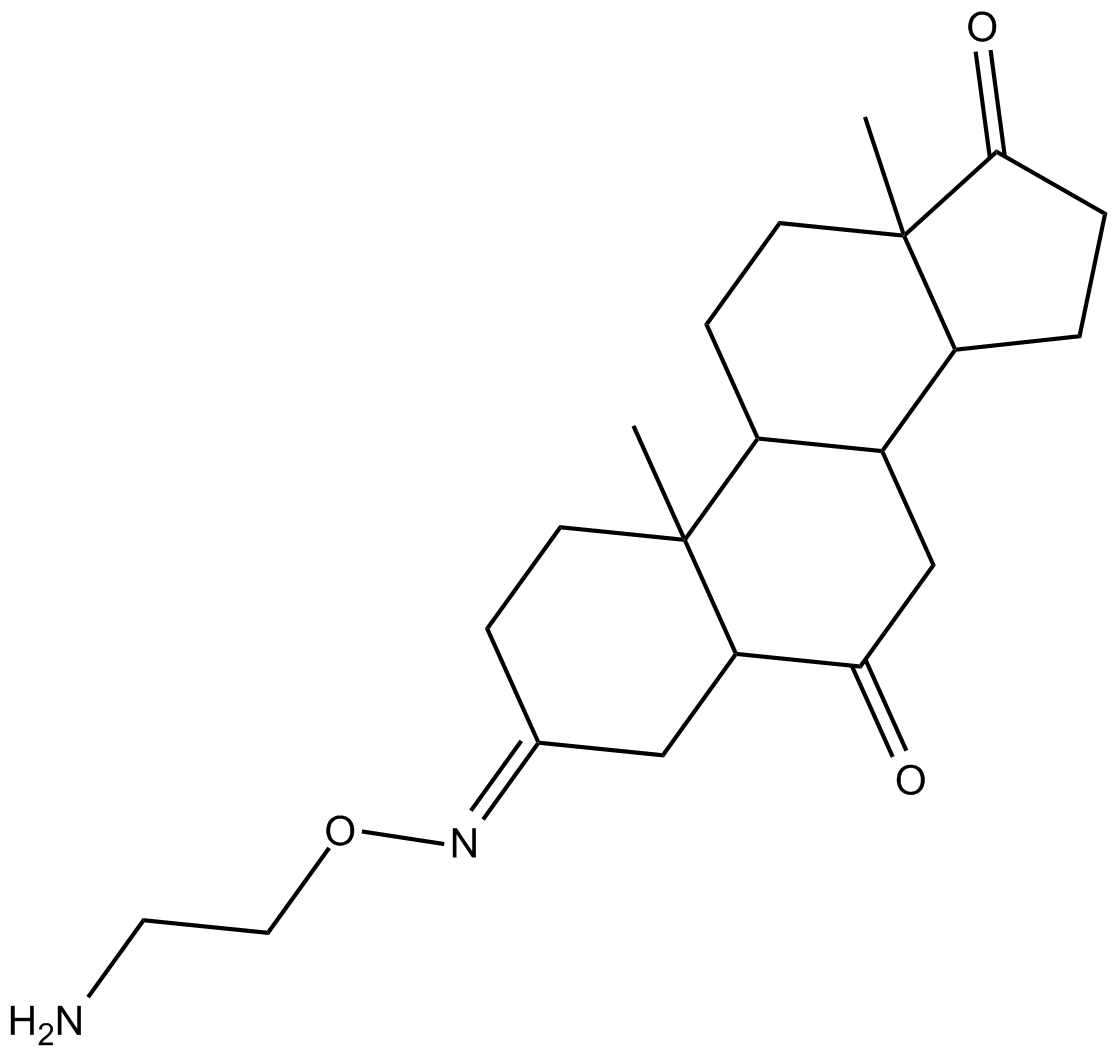
Cas No.: 203737-93-3
Sample solution is provided at 25 µL, 10mM.
Description:
IC50 Value: 0.43 ± 0.15 μM (Na+/K+-ATPase activity from dog kidney) [1]
Istaroxime is a positive inotropic agent that mediates its action through inhibition of sodium/potassium adenosine triphosphatase (Na+/K+ ATPase). Istaroxime is an investigational drug originally patented and developed by the italian pharmaceutical company Sigma-Tau. Istaroxime is now under development for treatment of acute decompensated heart failure by CVie Therapeutics.
in vitro: PST2744 inhibited the Na+/K+-ATPase activity from dog kidney with an IC50 value of 0.43 ± 0.15 μM [1]. The transient inward current (I(TI)) induced by a Ca(2+) transient in the presence of complete Na(+)/K(+) pump blockade was inhibited (-43%) by PST2744 but not by digoxin [2].
in vivo: Intravenous infusion of 0.2 mg/kg/min PST2744 in anesthetized guinea pigs exerted an immediate and long-lasting inotropic effect (ED(80) of 1.89 +/- 0.37 mg/kg) without causing lethal arrhythmias up to a cumulative dose of 18 mg/kg [1]. Istaroxime intravenous infusion (0.11 mg/kg per min) significantly increased both indices of contraction and relaxation (fractional shortening, +18+/-3.7%; aortic flow rate, +19+/-2.9%; peak myocardial systolic velocity, +36+/-7%; circumferential fiber shortening, +24+/-4.1%; peak atrial flow velocity, +69+/-8.6%; isovolumic relaxation time, +19+/-6.9%; and peak myocardial early diastolic velocity, +42+/-12%) [3]. In 5 animals, PST-2744 effects were compared with dobutamine. Heart rates, PR intervals and QT intervals were unchanged following PST-2744 administration. PST-2744 increased contractility (+dP/dt) by 56% from 1881 +/- 282 mm Hg/s to 2939 +/- 734 mm Hg/s (P < 0.01) [4].
Clinical trial: HORIZON-HF: A Phase II Trial to Assess Hemodynamic Effects of Istaroxime in Patients With Worsening HF and Reduced LV Systolic Function. Phase 2
Review for Istaroxime
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
Review for Istaroxime
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















