Lusutrombopag (S-888711) |
| Catalog No.GC31669 |
Lusutrombopag (S-888711) is an orally bioavailable thrombopoietin (TPO) receptor agonist, used for treatment of chronic liver disease.
Products are for research use only. Not for human use. We do not sell to patients.
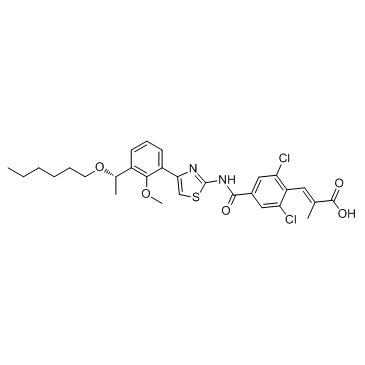
Cas No.: 1110766-97-6
Sample solution is provided at 25 µL, 10mM.
Lusutrombopag, an orally bioavailable, small molecule thrombopoietin receptor agonist, is approved for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure[1].
Lusutrombopag exhibits agonist activity for human thrombopoietin(TPO) receptor c-Mpl. Lusutrombopag promoted the proliferation of Ba/F3-hMpl cells. The 50% EC50 values of lusutrombopag Ba/F3-hMpl cells were 84.0, whereas lusutrombopag exhibited no proliferative activity in Ba/F3-mMpl cells. These results indicate that lusutrombopag promotes the proliferation of Ba/F3-hMpl cells via human c-Mpl. To investigate the signal transduction pathway of lusutrombopag, we evaluated the phosphorylation of JAK2, STAT3, STAT5 and p44/42 MAPK in Ba/F3-hMpl cells. Lusutrombopag phosphorylated these molecules similarly to rhTPO. These results suggest that lusutrombopag activates the same signal transduction pathways activated by rhTPO[2]
Lusutrombopag significantly increased circulating platelets in a dose-dependent manner during 21-day repeated oral administration in TPOR-Ki/Shi mice, was developed by replacing mouse Mpl with human-mouse chimera Mpl. Histopathological study of the TPOR-Ki/Shi mice on day 22 also revealed a significant increase in megakaryocytes in the bone marrow. These results indicate that lusutrombopag acts on human TPOR to upregulate differentiation and proliferation of megakaryocytic cells, leading to platelet production[2]
Lusutrombopag significantly increased the platelet count in all 31 patients with a mean increase of 31,000/µL.However, the increase in the platelet count after platelet transfusion was not statistically significant. When 13 patients repeated uses of lusutrombopag were counted platelet transfusion was not required in 82.1% (23/28) of treatments[3]
References:
[1]. Shirley M, McCafferty EH, et al. Lusutrombopag: A Review in Thrombocytopenia in Patients with Chronic Liver Disease Prior to a Scheduled Procedure. Drugs. 2019 Oct;79(15):1689-1695.
[2]. Yoshida H, Yamada H, et al. Development of a new knock-in mouse model and evaluation of pharmacological activities of lusutrombopag, a novel, nonpeptidyl small-molecule agonist of the human thrombopoietin receptor c-Mpl. Exp Hematol. 2018 Mar;59:30-39.e2.
[3]. Nomoto H, Morimoto N, et al. Lusutrombopag is effective and safe in patients with chronic liver disease and severe thrombocytopenia: a multicenter retrospective study. BMC Gastroenterol. 2020 Dec 14;20(1):427.
Average Rating: 5 (Based on Reviews and 31 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















