Mafenide Acetate |
| Catalog No.GC15799 |
sulfonamide-type medication
Products are for research use only. Not for human use. We do not sell to patients.
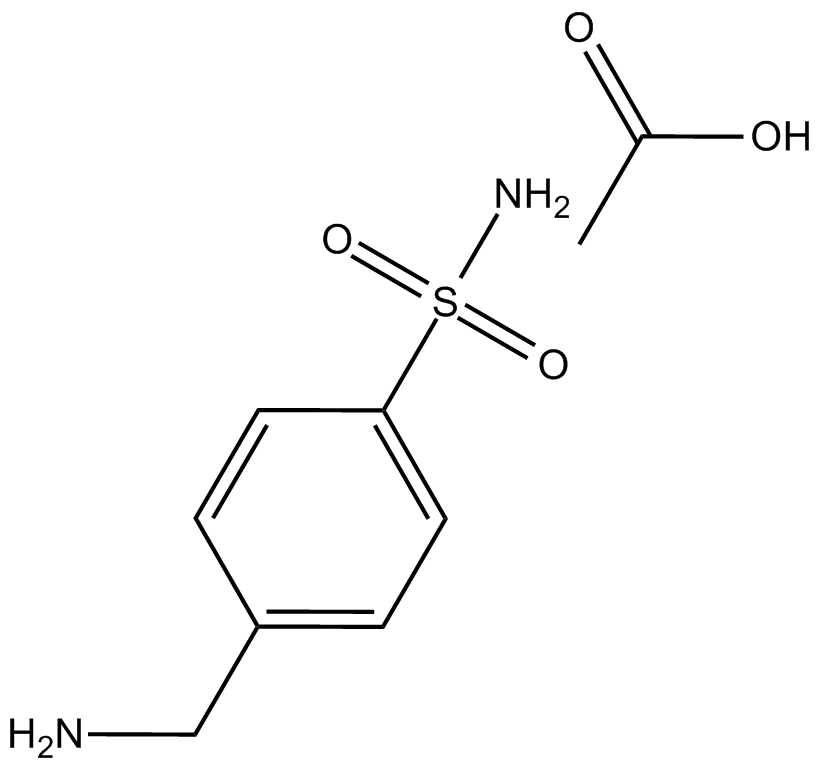
Cas No.: 13009-99-9
Sample solution is provided at 25 µL, 10mM.
Mafenide Acetate is a sulfonamide-type medication.Target: OthersMafenide is a sulfonamide-type medication. Mafenide works by reducing the bacterial population present in the avascular tissues of burns and permits spontaneous healing of deep partial-thickness burns. It is used to treat severe burns. It is used topically as an adjunctive therapy for second- and third-degree burns. It is bacteriostatic against many gram-positive and gram-negative organisms, including Pseudomonas aeruginosa. Some sources state that mafenide is more appropriate for non-facial burns, while chloramphenicol/prednisolone or bacitracin are more appropriate for facial burns [1-3].
References:
[1]. Siuda, J.F. and C.D. Cihonski, New compounds: carbamate derivatives of mafenide (homosulfanilamide). J Pharm Sci, 1972. 61(11): p. 1856-7.
[2]. Haynes, B.W., Jr., Mafenide acetate in burn treatment. N Engl J Med, 1971. 284(23): p. 1324.
[3]. Haik, J., et al., Burn care standards in Israel: lack of consensus. Burns, 2005. 31(7): p. 845-9.
Average Rating: 5 (Based on Reviews and 9 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















