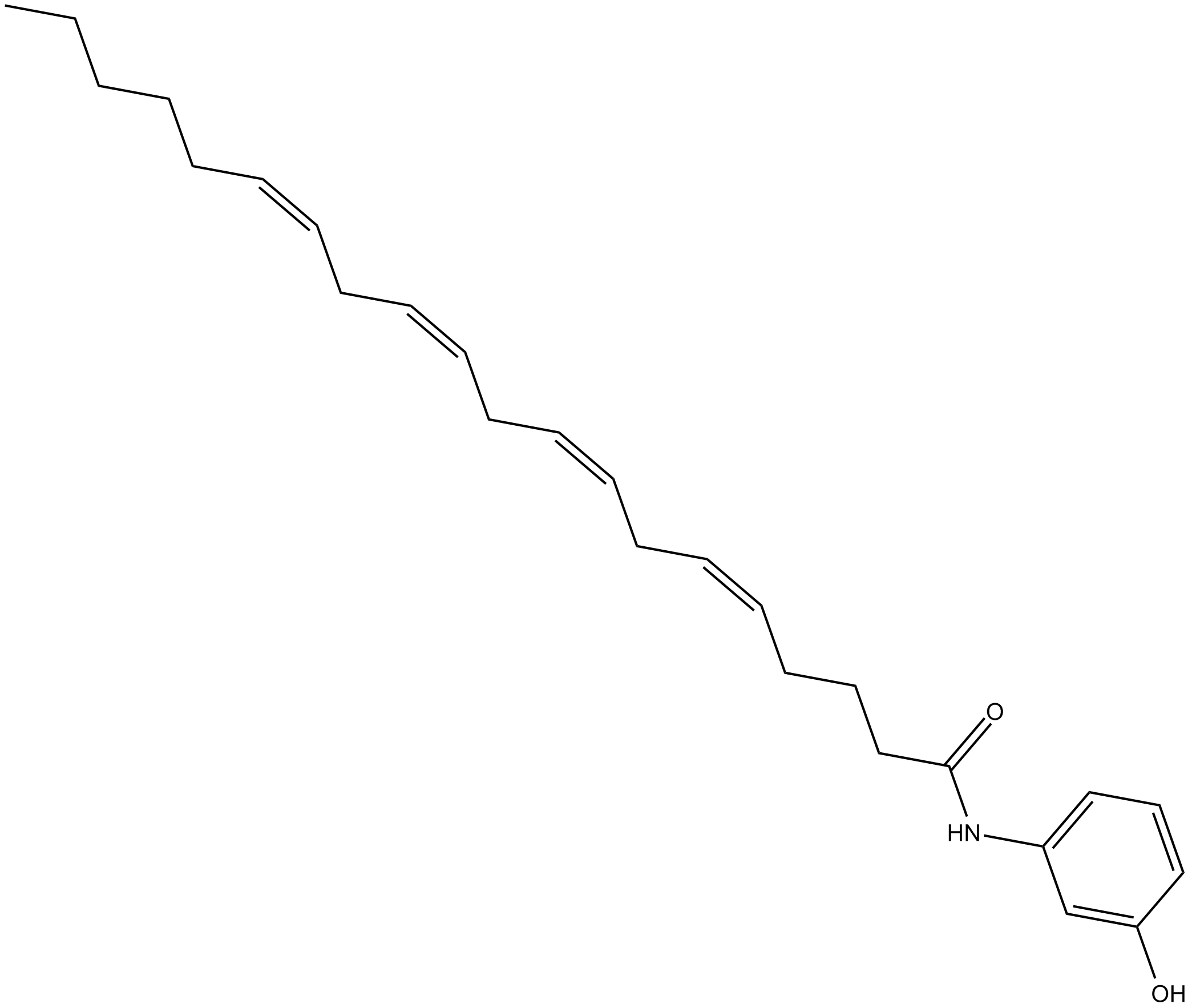
N-(3-hydroxyphenyl)-Arachidonoyl amide (Synonyms: 3-HPAA) |
| Catalog No.GC15372 |
selective, irreversible inhibitor of COX-2
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 183718-75-4
Sample solution is provided at 25 µL, 10mM.
IC50: 2 μM
N-(3-hydroxyphenyl) -Arachidonoyl amide, also known as 3-HPAA, is an analog of AM404 (N-(4-hydroxyphenyl)-arachidonoyl amide), a selective inhibitor of carrier-mediated transport of arachidonoyl ethanolamide. 3-HPAA, metabolized by both cyclooxygenase (COX) -1 and COX-2, is an irreversible and selective inhibitor of COX-2 with an IC50 value of 2 M. 3-HPAA can be efficiently oxygenated to prostaglandin and hydroxyeicosatetraenoate products by prostaglandin endoperoxide synthase (PGHS) -2. It appears that 3-HPAA can be converted by PGHS-1 in a similar manner.
COX enzymes play elaborate roles in human physiology and pathology, involving neuronal, immune, renal, cardiovascular, gastrointestinal, and reproductive systems. COX enzymes are blocked by aspirin and a wide variety of other non-steroidal anti-inflammatory drugs, which makes them clinically important [1]. COX-2, overexpressed in cancer cells, promotes tumorigenesis and induces neo-angiogenesis. Additionally, it plays an important role in inflammation and pyrexia.
In vitro: Up to now, in vitro study of 3-HPAA is still in the development stage.
In vivo: Up to now, in vivo study of 3-HPAA is still in the development stage.
Reference:
[1]. Fitzpatrick, F. Cyclooxygenase Enzymes: Regulation and Function. Current Pharmaceutical Design. 2004; 10(6): 577-588.
Average Rating: 5 (Based on Reviews and 15 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















