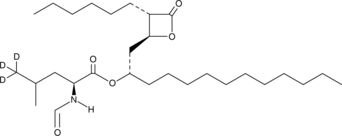
Orlistat-d3 (Synonyms: (–)-Tetrahydrolipstatin-d3) |
| Catalog No.GC49236 |
An internal standard for the quantification of orlistat
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1356930-46-5
Sample solution is provided at 25 µL, 10mM.
Orlistat-d3 is intended for use as an internal standard for the quantification of orlistat by GC- or LC-MS. Orlistat is a digestive lipase inhibitor.1,2,3 It inhibits diacylglycerol lipase α (DAGLα), DAGLβ, α/β-hydrolase domain-containing protein 12 (ABHD12), ABHD16A, and platelet-activating factor acetylhydrolase (PAF-AH; IC50s = 0.06, 0.1, 0.08, 0.03, and 0.05 µM, respectively), as well as pancreatic lipase and hormone-sensitive lipase (IC50s = 0.65 and 2.1 µg/ml, respectively) but does not inhibit fatty acid amide hydrolase (FAAH) or KIAA1363 (IC50s = >100 µM for both). Orlistat decreases ionomycin-induced production of the endocannabinoid 2-arachidonoyl glycerol (2-AG) in N18TG2 murine neuroblastoma cells when used at a concentration of 1 µM.4 It also inhibits fatty acid synthase (FASN; Kiapp = ~0.1 µM for the human enzyme) and the proliferation of PC3 prostate cancer cells in a concentration-dependent manner.5 Orlistat (10 mg/kg) decreases serum cholesterol levels and total body weight in a mouse model of obesity induced by a high-fat diet.6 Formulations containing orlistat have been used in the treatment of adult obesity.
1.Bisogno, T., Howell, F., Williams, G., et al.Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brainJ. Cell Biol.163(3)463-468(2003) 2.Hoover, H.S., Blankman, J.L., Niessen, S., et al.Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profilingBioorg. Med. Chem. Lett.18(22)5838-5841(2008) 3.Bustanji, Y., Issa, A., Mohammad, M., et al.Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituentsJ. Med. Plant Res.4(21)2235-2242(2010) 4.Bisogno, T., Cascio, M.G., Saha, B., et al.Development of the first potent and specific inhibitors of endocannabinoid biosynthesisBiochim. Biophys. Acta1761(2)205-212(2006) 5.Kridel, S.J., Axelrod, F., Rozenkrantz, N., et al.Orlistat is a novel inhibitor of fatty acid synthase with antitumor activityCancer Res.64(6)2070-2075(2004) 6.Ji, W., Zhao, M., Wang, M., et al.Effects of canagliflozin on weight loss in high-fat diet-induced obese micePLoS One12(6)e0179960(2017)
Average Rating: 5 (Based on Reviews and 32 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















