Peroxynitrite (Synonyms: Sodium Peroxynitrite) |
| Catalog No.GC44602 |
Formed in vivo by the reaction of NO with superoxide
Products are for research use only. Not for human use. We do not sell to patients.
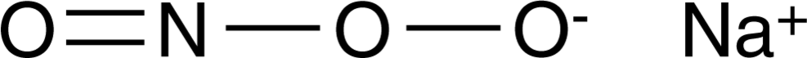
Cas No.: 14042-01-4
Sample solution is provided at 25 µL, 10mM.
Peroxynitrite is formed in vivo by the reaction of NO with superoxide.[1],[2],[3] It is a powerful oxidizing agent that can initiate lipid peroxidation, oxidize sulfhydryls, and nitrate the aromatic residues of proteins.
For long term storage, we suggest that peroxynitrite be stored as supplied at -80°C. It will be stable for at least three months.
Peroxynitrite is supplied as a solution in 0.3 M NaOH. Peroxynitrite is highly unstable and slowly decomposes even at -80°C but not to any significant extent within one month. The half-life of peroxynitrite in alkaline solutions at room temperature is about 5 hours. Peroxynitrite decomposes instantaneously under acidic conditions and the half-life at pH 7.4 is only few seconds [3]. Further dilutions of the stock solution can be performed using cold 0.3 M NaOH. We recommend that the actual concentration of peroxynitrite be measured following the procedure given below before using it in any experiments:
Thaw the peroxynitrite solution carefully and keep it on ice. Dilute an aliquot of the stock solution 40-fold with cold 0.3 M NaOH (e.g. add 25 μl of the stock to 975 μl of 0.3 M NaOH) and measure the absorbance at 302 nm with 0.3 M NaOH as blank. Concentration of the stock solution can be calculated using the extinction coefficient for peroxynitrite(1670 M-1cm-1).
Reference:
[1]. Pryor, W.A., and Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. American Journal of Physiology 268, L699-L722 (1995).
[2]. Beckman, J.S., and Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am. J. Physiol. 271(5 Pt 1), C1424-C1437 (1996).
[3]. Koppenol, W.H., Moreno, J.J., Pryor, W.A., et al. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chemical Research in Toxicology 5, 834-842 (1992).
Average Rating: 5 (Based on Reviews and 14 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















