SB 203580 (Synonyms: SB 203580; RWJ 64809) |
| Catalog No.GC13595 |
SB 203580 is a specific inhibitor of p38-MAPK (Mitogen-activated Protein Kinase) pathway .
Products are for research use only. Not for human use. We do not sell to patients.
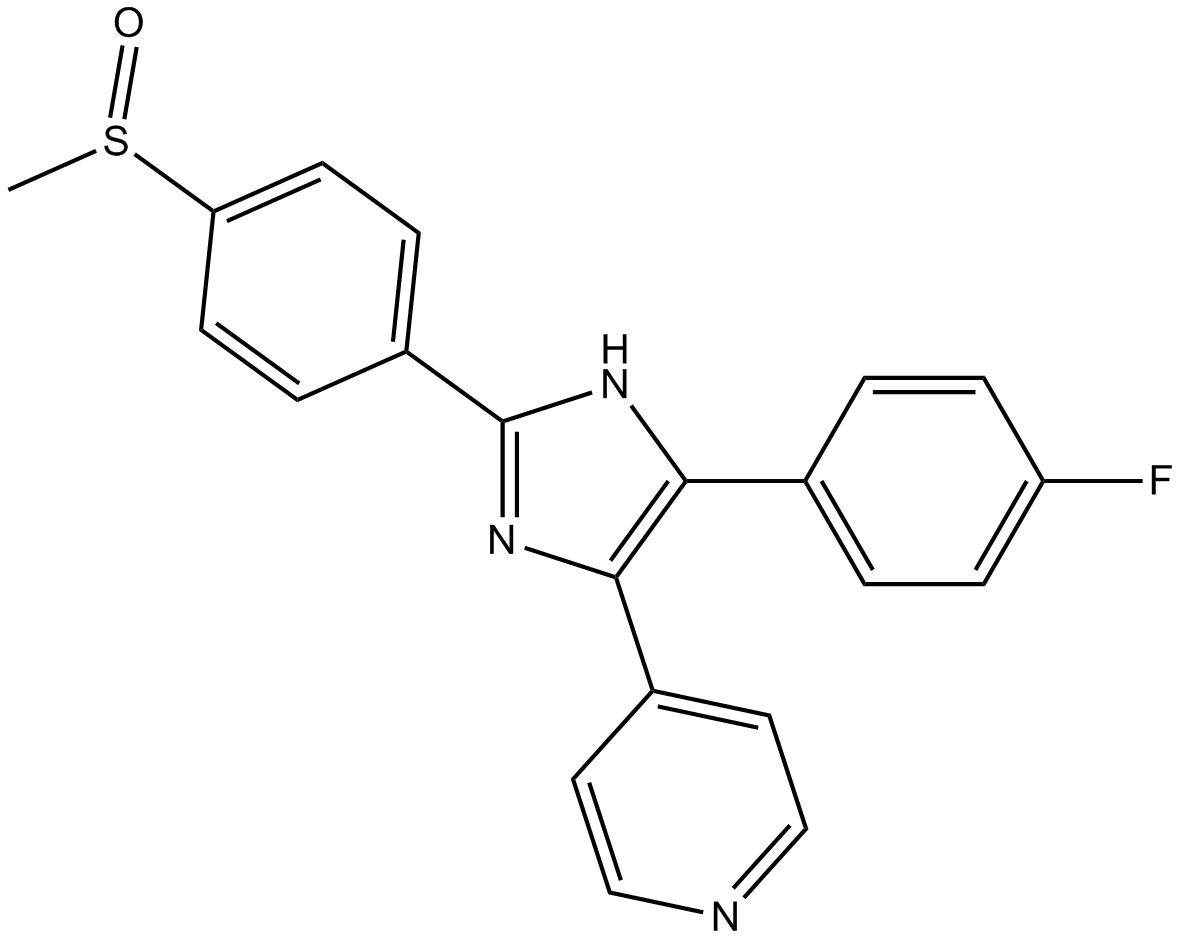
Cas No.: 152121-47-6
Sample solution is provided at 25 µL, 10mM.
SB 203580 is a specific inhibitor of p38-MAPK (Mitogen-activated Protein Kinase) pathway [1,2]. SB 203580 inhibits p38 kinase in a manner competitive with ATP with a Ki of 21 nm [2].
SB 203580 inhibit the proliferation of human breast cancer cell line MDA-MB-231 with the IC50 value of was 85.1 µM [3]. SB 203580 inhibited IL-10 production by monocytic WEHI 274.3 cells expressing WT-p38α MAPK in a dose-dependent manner with greater than 95% inhibition at 5 µm and with an IC50 of 0.1µM [4]. IL-2-induced proliferation of primary human T cells, murine CT6 T cells, or BAF F7 B cells is prevented by the p38 MAP kinase inhibitor SB 203580 with an IC50 of 3-5µM [5].
SB-203580 demonstrated moderate to high clearance in all species tested in vivo, with non-linear elimination observed in the rat at plasma concentrations > 1000ng ml -1. Although good solution bioavailability was observed in non-rodents (78% in dog, 32% in monkey), lower and more variable bioavailability was observed in the rat and mouse (3-48%) [6]. SB 203580 treatment significantly improve the white blood cell (WBC) and platelet counts decreased significantly in the DENV-infected mice, suggesting leucopenia and thrombocytopenia, respectively [7].
References:
[1]. Barancik M, Bohacova V, Kvackajova J, Hudecova S, Krizanova O, Breier A: SB 203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci. 2001, 14: 29-36. 10.1016/S0928-0987(01)00139-7.
[2]. Peter R. Young, Megan M. McLaughlin, Sanjay Kumar, et al. Pyridinyl Imidazole Inhibitors of p38 Mitogen-activated Protein Kinase Bind in the ATP Site. The Journal of Biological Chemistry, 1997, 272(18): 12116-12121.
[3]. Duzgun SA, Yerlikaya A, Zeren S, Bayhan Z, Okur E, Boyaci I. Differential effects of p38 MAP kinase inhibitors SB 203580 and SB202190 on growth and migration of human MDA-MB-231 cancer cell line. Cytotechnology. 2017;69(4):711-24.
[4]. Guo, X., R.E. Gerl, and J.W. Schrader. 2003. Defining the involvement of p38alpha MAPK in the production of anti- and proinflammatory cytokines using an SB 203580-resistant form of the kinase. J. Biol. Chem. 278:22237-22242.
[5]. Lali, F. V., Hunt, A. E., Turner, S. J., and Foxwell, B. M. The pyridinyl imidazole inhibitor SB 203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J. Biol. Chem.,275: 7395-7402,?2000
[6]. Ward KW, Prokscht JW, Azzaranot LM, et al. Preclinical pharmacokinetics of SB-203580, a potent inhibitor of p38 mitogen-activated protein kinase. Xenobiotica 2001; 31: 783-97
[7]. Sreekanth GP, Chuncharunee A, Sirimontaporn A, Panaampon J, Noisakran S, Yenchitsomanus PT, et al. SB 203580 modulates p38 MAPK signaling and Dengue virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2 phosphorylation. PLoS One. 2016;11:e0149486.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *










