Solifenacin succinate (Synonyms: YM-67905) |
| Catalog No.GC17182 |
Muscarinic receptor antagonist
Products are for research use only. Not for human use. We do not sell to patients.
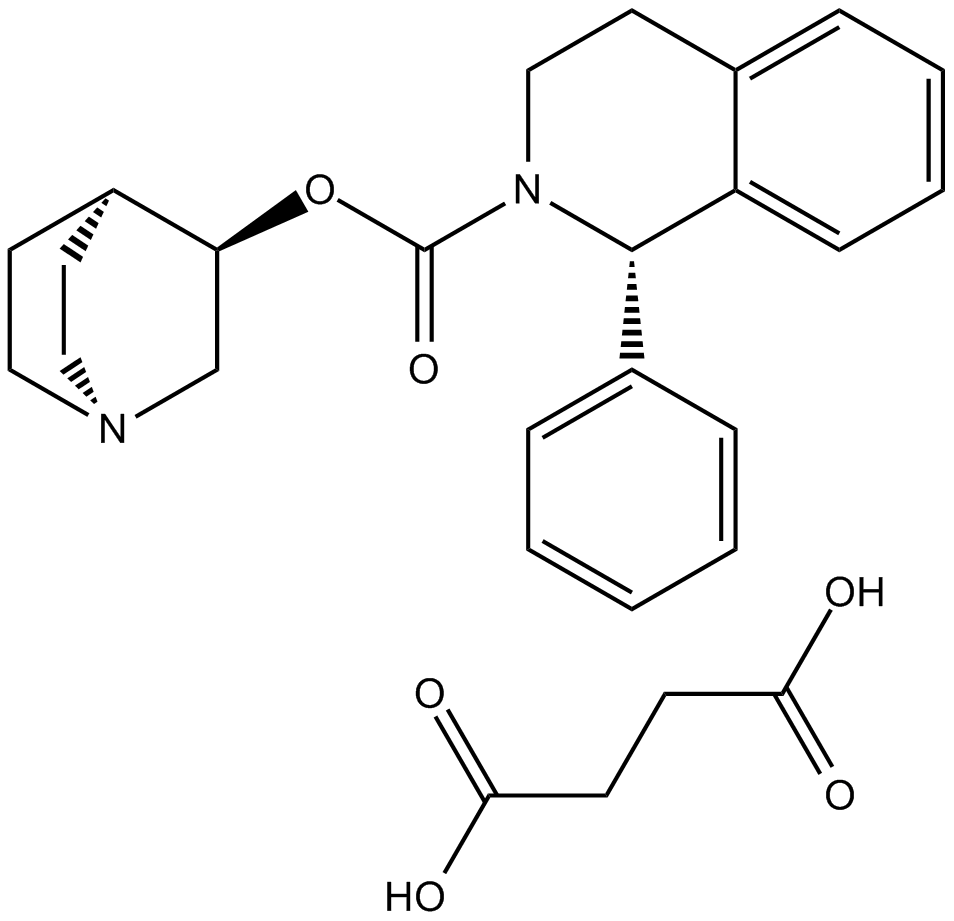
Cas No.: 242478-38-2
Sample solution is provided at 25 µL, 10mM.
Solifenacin succinate is a quinuclidine and tetrahydroisoquinoline derivative and selective M3 muscarinic antagonist. It is used as a urological agent in the treatment of overactive bladder[1].
M3 muscarinic receptor is a muscarinic acetylcholine receptor encoded by the human gene CHRM3. Muscarinic M3 receptors are involved in various cellular responses, including breakdown of phosphoinositides, inhibition of adenylate cyclase and modulation of potassium channels through the action of G proteins[2].
In vitro: In radioligand receptor binding assay, the Kivalues of solifenacin for human muscarinic M1, M2, M3, M4and M5receptors were 26, 170, 12, 110 and 31 nM, respectively. In isolated rat urinary bladder, solifenacin competitively antagonized carbachol-induced contractions, with a pA2value of 7.44±0.09[3].In bladder smooth muscle cells and salivary gland cells isolated from rats, solifenacin and the other antimuscarinic drugs inhibited carbachol-induced increases in intracellular Ca2+levels in a concentration-dependent manner. ThepKi was 8.12for bladder smooth muscle cells, 3.6-fold more potent than that for salivary gland cells (pKi=7.57) [1].
In vivo: In anesthetized rats, solifenacin dose-dependently inhibited carbachol-induced intravesical pressure elevation and salivary secretion, and exhibited selectivity (3.7- to 6.5-fold) for urinary bladder over salivary gland [1]. In anesthetized rats, solifenacin and oxybutynin increased the maximum bladder capacity in a dose-dependent manner and also decreased the maximum intravesical pressure [3].In healthy young men, multidose study evaluated doses. In the single-dose of solifenacin succinate (5-, 10-, 20-, and 30-mg), mean time to maximal concentration and elimination half-life ranged from 3.3 to 4.8 and from 40.2 to 57.6 hours, respectively.In the multidose study, the ranges were 2.9 to 5.8 and 45.0 to 64.8, respectively. The single-dose administration was well tolerated. The common adverse events were dry mouth, blurred vision, and headache [4].
Clinical trials: In this phase 3 trial in patients with symptoms related to overactive bladder, treatment with solifenacin (5 mg or 10 mg, once daily) significantly improved all the major symptoms of overactive bladder including urgency, frequency and incontinence. Solifenacin (10 mg) decreased the frequency of nocturia. Solifenacin therapy has been associated with a favorable tolerability profile and low incidence of dry mouth, especially at the 5 mg starting dose [5].
References:
[1]. Ohtake A, Ukai M, Hatanaka T, et al. In vitro and in vivo tissue selectivity profile of solifenacin succinate (YM905) for urinary bladder over salivary gland in rats[J]. European journal of pharmacology, 2004, 492(2): 243-250.
[2]. Yang J, Williams J A, Yule D I, et al. Mutation of carboxyl-terminal threonine residues in human m3 muscarinic acetylcholine receptor modulates the extent of sequestration and desensitization[J]. Molecular pharmacology, 1995, 48(3): 477-485.
[3]. Ohtake A, Saitoh C, Yuyama H, et al. Pharmacological characterization of a new antimuscarinic agent, solifenacin succinate, in comparison with other antimuscarinic agents[J]. Biological and Pharmaceutical Bulletin, 2007, 30(1): 54-58.
[4]. Smulders R A, Krauwinkel W J, Swart P J, et al. Pharmacokinetics and safety of solifenacin succinate in healthy young men[J]. The Journal of Clinical Pharmacology, 2004, 44(9): 1023-1033.
[5]. Cardozo L, Lisec M, Millard R, et al. Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder[J]. The Journal of urology, 2004, 172(5): 1919-1924.
Average Rating: 5 (Based on Reviews and 17 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















