Sphingosine (d17:1) (Synonyms: D-erythro-Sphingosine C-17) |
| Catalog No.GC44932 |
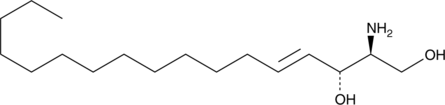
Sphingosine is an amino alcohol most commonly characterized by an 18-carbon unsaturated hydrocarbon chain sphingosine (d18:1).
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 6918-48-5
Sample solution is provided at 25 µL, 10mM.
Sphingosine is an amino alcohol most commonly characterized by an 18-carbon unsaturated hydrocarbon chain sphingosine (d18:1) . However, the hydrocarbon chain length of sphingosine, and the related dihydrosphingosine, can vary from 12-26 carbons in mammalian tissues. Sphingosine (d17:1) is a naturally-occurring but uncommon form of sphingosine, accounting for approximately 13% of the sphingosine in human skin. It can be phosphorylated by sphingosine kinases to produce C-17 sphingosine-1-phosphate. More commonly, sphingosine C-17 is used as an internal standard in the analysis of sphingoid compounds by chromatographic or spectrometric methods.
Average Rating: 5 (Based on Reviews and 35 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















