Terephthalic acid |
| Catalog No.GC30708 |
Terephthalic acid is one isomer of the three phthalic, a precursor to the polyester PET, used to make clothing and plastic bottles.
Products are for research use only. Not for human use. We do not sell to patients.
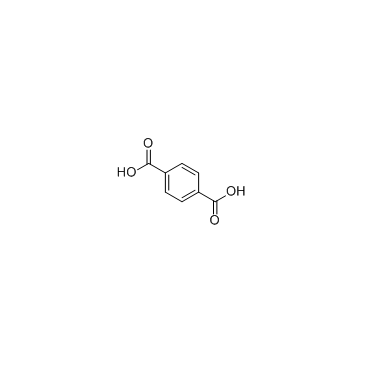
Cas No.: 100-21-0
Sample solution is provided at 25 µL, 10mM.
Terephthalic acid is one isomer of the three phthalic, a precursor to the polyester PET, used to make clothing and plastic bottles.
[1]. Heck HD, et al. The induction of bladder stones by terephthalic acid, dimethyl terephthalate, and melamine (2,4,6-triamino-s-triazine) and its relevance to risk assessment. Regul Toxicol Pharmacol. 1985 Sep;5(3):294-313.
Average Rating: 5 (Based on Reviews and 19 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















